
����Ŀ����1�����г������ʣ�
A��̼������ B����˹ƥ�� C�������� D���������� E�����ʻ�
������ĸ��գ�
�����н�����ʹ��Ч����________������ֱ�ӽ�������ѪҺ������������________����������ʳƷ����������________����������ʳƷ��ɫ������________��������������θ�����Ŀ��������________��
��2��������������������
��������������У�����Ҫ����ָ����_________��
A�������������ĺ��� B��NO2Ũ�� C��SO2Ũ�� D��CO2Ũ��
���ڴ��������ķ�ˮʱ�����ȼ�����������������____________________________________����ͨ��������������ȣ���������____________________________________________��
������β����Ҫ�ô�ת���������ж������ŷš�д������β����CO��NO��������ɿɲ������ѭ������������Ļ�ѧ����ʽ_________________________________________��
����������Ҫ���ദ������ͼ��ʾ�����������־�ĺ�����__________________��

��3����ѧ��������й�����
��ʯīϩ(��ͼ)������̫���ܵ�صĵ缫,������Ҫ������ʯīϩ��__________________�ԡ�

�ڻ������̽����г��õ�ˮ�����������ֲĵȡ�����ˮ��Ͳ������õ���ԭ����___________________��
�������������лᷢ���绯ѧ��ʴ�����һ������������ʩ_____________________________________��
���𰸡�B C D E A D ����������ˮ���� ����ɱ�� 2NO+2CO ![]() N2 + 2CO2 �ɻ��ջ�ѭ������ ���� ʯ��ʯ ˢ����Ⱥ�����
N2 + 2CO2 �ɻ��ջ�ѭ������ ���� ʯ��ʯ ˢ����Ⱥ�����
��������
��1�������ʵ����ʺ���;��֪��
A��̼�����ƿ��Ժ�θ�ᣨ���ᣩ��Ӧ������������θ�����Ŀ������
B����˹ƥ�־��н�����ʹ��Ч��
C����������������Ѹ���������ͷ��������ʿ�ֱ�ӽ�������ѪҺ����������
D���������ƿ�����ʳƷ��������
E�����ʻƳ�����ʳƷ��ɫ����
��2��������������������ǿ���������NO2��SO2����Ⱦ���������ʣ�������̼Ϊ�����壻
����������ˮʱ�������������ˮ�����������������壬���������Կ��Ծ�ˮ��������������Ⱦ���ǿ�����ԣ�������ɱ����
��CO��NO��������ɿɲ������ѭ������������ӦΪ�����Ͷ�����̼����ƽ���û�ѧ����ʽ��
����ͼ��ʾ�����������־�ĺ����ǿɻ��ջ�ѭ��������
��3����ʯīϩ����ʮ�����õ�ǿ�ȡ����͡����硢���ȡ���ѧ�����ܣ����缫��������ʯīϩ�ĵ����ԣ�
������ˮ���������ʯ��ʯΪ��Ҫԭ�ϣ�����������ԭ���Ǵ��ʯ��ʯ��ʯӢ�����Զ��õ�ʯ��ʯ��
�۷�ֹ������ʴ��õķ������ڽ����ı��渲�DZ����������ڸ�������Ϳ�ͻ�����ȣ�ʹ�����������ˮ�������Դﵽ��ֹ������ʴ��Ŀ����
��1���������Ϸ���5�����ʣ�
�����н�����ʹ��Ч���ǰ�˹ƥ������ѡB��
����ֱ�ӽ�������ѪҺ����������������������ѡC��
��������ʳƷ���������DZ�����������ѡD��
��������ʳƷ��ɫ���������ʻ�����ѡE��
������������θ�����Ŀ��������̼�����ƣ���ѡA��
��2���ٿ���������NO2��SO2������Ⱦ���������ʣ�������̼Ϊ�����壬�ʿ�����������У�����Ҫ����ָ����CO2Ũ�ȣ�
��ˣ�������ȷ���ǣ�D��
����������ˮʱ�������������ˮ�����������������壬���������Կ��Ծ�ˮ�����������ǻ�������ˮ����������������Ⱦ���ǿ�����ԣ�������ɱ����
��ˣ�������ȷ���ǣ���������ˮ��������ɱ����
��CO��NO��������ɿɲ������ѭ������������ӦΪ�����Ͷ�����̼���ʻ�ѧ����ʽΪ��2NO+2CO ![]() N2 + 2CO2��
N2 + 2CO2��
��ˣ�������ȷ���ǣ�2NO+2CO ![]() N2 + 2CO2
N2 + 2CO2
����ͼ��ʾ�����������־�ĺ����ǿɻ��ջ�ѭ��������
��ˣ�������ȷ���ǣ��ɻ��ջ�ѭ��������
��3����ʯīϩ����ʮ�����õ�ǿ�ȡ����͡����硢���ȡ���ѧ�����ܣ����缫��������ʯīϩ�ĵ����ԣ�
��ˣ�������ȷ���ǣ����磻
������ˮ���������ʯ��ʯΪ��Ҫԭ�ϣ�����������ԭ���Ǵ��ʯ��ʯ��ʯӢ�����Զ��õ�ʯ��ʯ��
��ˣ�������ȷ���ǣ�ʯ��ʯ��
�۷�ֹ������ʴ��õķ������ڽ����ı��渲�DZ����������ڸ�������Ϳ�ͻ�����ȣ�ʹ�����������ˮ�������Դﵽ��ֹ������ʴ��Ŀ�ģ�
��ˣ�������ȷ���ǣ�ˢ����Ⱥ����𰸡�


 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����![]() Ϊ�����ӵ�������ֵ������������ȷ����
Ϊ�����ӵ�������ֵ������������ȷ����![]() ����
����![]()
A. ��״���£�22.4LCO��CO2�Ļ�����У���̼ԭ�ӵ���ĿΪNA
B. 14g����ʽΪC5H10�����У����е�̼̼˫������ĿΪ0.2NA
C. 0.1mol/L��CH3COONH4��Һ�У���笠�������ĿС��0.1NA
D. ��״����2.24LCl2ͨ������ˮ�л�NaOH��Һ�У�ת�Ƶ�����ĿΪ0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����:��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
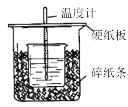
(1)��ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��_____________��
(2)�����ͼ����ʾ��װ�ý������飬��õ��к�����ֵ_______(����ƫ��ƫС����Ӱ����)��
(3)ʵ���и���60 mL 0.50 mol��L-1�����50 mL 0.55 mol��L-1NaOH��Һ���з�Ӧ����(2)��ʵ����ȣ������к���_________ (��������������������)��
��: (1)�������˻������������ȼ���DZ���(C3H8)������������˻���ȼ���DZ�ϩ(C3H6),��������ɵñ�ϩ����֪��C3H8(g) === CH4(g)��HC��CH(g)��H2(g) ��H1=+156.6 kJ��mol��1
CH3CH��CH2(g)=== CH4(g)�� HC��CH(g ) ��H2=+32.4 kJ��mol��1
��C3H8(g) === CH3CH��CH2(g)��H2(g) ��H =______________ kJ��mol��1��
(2)������ʱ����(N2H4)��ȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪32gN2H4(g)��ȫ����������Ӧ�ų�568kJ���������Ȼ�ѧ����ʽ�ǣ�____________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����VL����MgSO4��K2SO4�Ļ����Һ�ֳ����ȷݣ�һ�ݼ��뺬amolNaOH����Һ��ǡ��ʹþ������ȫ����Ϊ������þ����һ�ݼ��뺬bmolBaCl2����Һ��ǡ��ʹ�����������ȫ����Ϊ���ᱵ����ԭ�����Һ�м����ӵ����ʵ���Ϊ
A. (b-a)molB. (2b-a)molC. 2(b-a)molD. 2(2b-a)mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��״���£�1.92 gij��������Ϊ672 mL������������Է�������Ϊ______��
(2)��25 �桢101 kPa�������£�ͬ������CH4��A��������֮����15��8����A��Ħ������Ϊ________��
(3)������ͬ�ݻ����ܱ�����X��Y����25 ���£�X�г���a g A���壬Y�г���a g CH4���壬X��Y�ڵ�ѹǿ֮����4��11����A��Ħ������Ϊ________��
(4)��ͬ�����£������Ϊa��b��������Ϊa��b��H2��O2�Ļ�����壬��ƽ��Ħ�������ֱ���________��________��
(5)�ڱ�״���£�CO��CO2�Ļ�����干39.2 L������Ϊ61 g����������������ʵ���֮��Ϊ________mol��COռ�������________%��
(6)��ij�¶�ʱ��һ������Ԫ��A���⻯��AH3���ں��º�ѹ���ܱ���������ȫ�ֽ�Ϊ������̬���ʣ���ʱ�����������Ϊԭ����7/4����A���ʵķ���ʽΪ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���ɶ�����Ԫ����ɵ�һЩ���ʼ��仯����֮���ת����ϵͼ���������ʾ�йص�һ�ַ�Ӧ��������ijЩ�����Ѿ���ȥ��������A��B��D�ڳ����¾�Ϊ��ɫ�̼�����ζ�����壬C��ʹʪ��ĺ�ɫʯ����ֽ���������壬M���������ɫҺ�塣
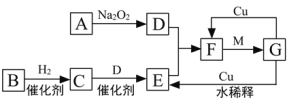
��1������G�Ļ�ѧʽ��__________________��
��2������B��������____________________��
��3��д��A��D�Ļ�ѧ����ʽ��_______________________________________________��
F��G�Ļ�ѧ����ʽ��______________________________________________________��
G��E�����ӷ���ʽ��______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ī˾͡(Bendamustine)��һ�ֿ���ҩ�����Ī˾͡��һ�ֺϳ�·�����£�

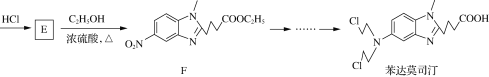
(1) D�������������������____(������)��
(2) A��B�ķ�Ӧ����Ϊ____��
(3) E�ķ���ʽΪC12H13N3O4��д��E�Ľṹ��ʽ��__________��
(4) G��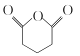 ��ͬ���칹�壬G�ܷ���������Ӧ��������ֻ��2�ֲ�ͬ��ѧ�������⡣д��һ�ַ���������G�Ľṹ��ʽ��__________��
��ͬ���칹�壬G�ܷ���������Ӧ��������ֻ��2�ֲ�ͬ��ѧ�������⡣д��һ�ַ���������G�Ľṹ��ʽ��__________��
(5)��֪��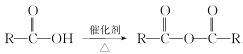 ����д����
����д����![]() �Ҵ�Ϊԭ���Ʊ�
�Ҵ�Ϊԭ���Ʊ�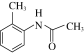 �ĺϳ�·������ͼ(���Լ����ã��ϳ�·������ͼʾ�����������)__________________________��
�ĺϳ�·������ͼ(���Լ����ã��ϳ�·������ͼʾ�����������)__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��6.02��1023�����������ӵ����ʵ�����________mol����Ħ������Ϊ________��
��2���ڱ�״���£�0.01 molij���������Ϊ0.44 g�����������ܶ�Ϊ________g��L1������С�������λ�������������Է�������Ϊ________��
��3���ڱ�״���£���CO��CO2��ɵĻ������Ϊ6.72 L������Ϊ12 g���˻������CO��CO2���ʵ���֮����________��CO�����������________��CO������������________��C��Oԭ�Ӹ�������________����������ƽ����Է���������________���ܶ���________g��L1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������������( )
A.�Ͻ�����п��Ժ��зǽ���Ԫ��
B.����ϳɲ�����ָ������ά�����Ϻ���
C.������ɱ���ǵ��״����������Ϊ�����еĵ��������ȱ���
D.�������������ϼ�װ����ת��������Ϊ�˼����к�������ŷ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com