
ÓĆĶŃ֬Ǝ°ü×”Ō¼0.2 g¹żŃõ»ÆÄĘ·ŪÄ©£¬ÖĆÓŚŹÆĆŽĶųÉĻ£¬ĶłĶŃ֬ƎÉĻµĪĖ®£¬æɹŪ²ģµ½ĶŃ֬Ǝ¾ēĮŅČ¼ÉÕĘšĄ“”£
(1)ÓÉÉĻŹöŹµŃéĻÖĻóĖłµĆ³öµÄÓŠ¹Ų¹żŃõ»ÆÄĘøśĖ®·“Ó¦µÄ½įĀŪŹĒ£ŗµŚŅ»£¬ÓŠŃõĘųÉś³É£»µŚ¶ž£¬________”£¹żŃõ»ÆÄĘøśĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½_______________________________________________________________”£
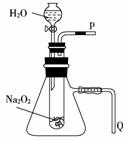
(2)Ä³ŃŠ¾æŠŌѧĻ°Š”×éÄāÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃ飬ŅŌÖ¤Ć÷ÉĻŹö½įĀŪ”£ÓĆŅŌŃéÖ¤µŚŅ»Ģõ½įĀŪµÄŹµŃé·½·Ø¼°ĻÖĻóŹĒ__________________”£
ÓĆŅŌŃéÖ¤µŚ¶žĢõ½įĀŪµÄŹµŃé·½·Ø¼°ĻÖĻóŹĒ__________________”£
”¾½āĪö”æ””¹żŃõ»ÆÄĘÓėĖ®·“Ӧɜ³ÉO2£¬²¢ĒŅ·“Ó¦·ÅČČ£¬“ļµ½ĶŃ֬ƎµÄČ¼µć£¬Ź¹ĆŽ»Ø¾ēĮŅČ¼ÉÕ”£æÉŌŚp“¦ŹÕ¼Æ²śÉśµÄĘųĢ壬²¢ÓĆ“ųÓŠÓą½żµÄľĢõ¼ģŃ飬ŌŚQ“¦·ÅŅ»Ź¢ÓŠĖ®µÄÉÕ±£¬Čō·“Ó¦·ÅČČ£¬Ōņ׶ŠĪĘæÖŠĘųĢåŹÜČČÅņÕĶ£¬Ė®ÖŠ»įÓŠĘųÅŻ²śÉś”£
”¾“š°ø”æ””(1)·“Ó¦·ÅČČ””2Na2O2£«2H2O===4NaOH£«O2”ü””(2)ŌŚp“¦ŹÕ¼ÆŅ»ŹŌ¹ÜĘųĢ壬½«“ųÓŠÓą½żµÄľĢõ·ÅŌŚŹŌ¹ÜæŚ£¬ČōľĢõø“Č¼£¬ŌņÖ¤Ć÷Éś³ÉµÄĘųĢåŹĒO2””ŌŚQ“¦·ÅŅ»Ź¢ÓŠĖ®µÄÉÕ±£¬ČōQ“¦ÓŠĘųÅŻ²śÉś£¬ŌņÖ¤Ć÷·“Ó¦·Å³öČČĮæ


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
12.4 g Na2Rŗ¬Na£«0.4 mol£¬ŌņNa2RµÄĦ¶ūÖŹĮæĪŖ________£¬RµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ________£®ŗ¬RµÄÖŹĮæĪŖ1.6 gµÄNa2R£¬ĘäĪļÖŹµÄĮæĪŖ________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄĪļÖŹ A ”¢ B ”¢C ”¢D £¬¾ßÓŠČēĻĀ·“Ó¦¹ŲĻµ£ŗ
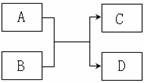 |
(1) Ķس£ČōAĪŖ»ĘĀĢÉ«µ„ÖŹ£¬BĪŖĪŽÉ«ŅŗĢ¬»ÆŗĻĪļ£¬ĒŅ0.1mol/LµÄCČÜŅŗpH=1£¬ŌņDĪļÖŹµÄ½į¹¹Ź½ĪŖ_____________
(2) ČōBĪŖ»ÆŗĻĪļ£¬ĒŅA”¢CĮ½ÖÖĪŽÉ«ĘųĢåĻąÓöŗó±äĪŖŗģ×ŲÉ«£¬ŌņBĪļÖŹČÜÓŚĖ®ŗóĻŌ¼īŠŌµÄŌŅņŹĒ__________________________________________________(ÓĆ»ÆѧÓĆÓļĖµĆ÷)
(3)ČōAĪŖµ„ÖŹ£¬CŗĶDĪŖ³£¼ūµÄŅ×Č¼ĘųĢ壬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____________________________________________________
(4) ČōAĪŖµ„ÖŹ£¬BĶس£ŹĒĪŽÉ«ŅŗĢ¬»ÆŗĻĪļ£¬×ĘÉÕD²śÉś»ĘÉ«»šŃę£¬Ōņ£ŗ
¢ŁČōB·Ö×Óŗ¬ÓŠ10øöµē×Ó£¬AÓėB·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ______________________________
¢ŚČōB·Ö×Óŗ¬ÓŠ18øöµē×Ó£¬ŌņA+B”śC+DµÄ»Æѧ·½³ĢŹ½ĪŖ_____________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŅŗĢåæÉŅŌÓĆĄ“±£“ę½šŹōÄʵďĒ(””””)
A£®Ė®”””””””””””””””””” B£®ÅØNaOHČÜŅŗ
C£®ĆŗÓĶ D£®CCl4(¦Ń>1 g”¤cm£3)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éÖŠµÄĮ½ÖÖĪļÖŹ×÷ÓĆŹ±£¬·“Ó¦Ģõ¼ž»ņ·“Ó¦ĪļÓĆĮæµÄøı䣬¶ŌÉś³ÉĪļƻӊӰĻģµÄŹĒ(””””)
A£®Na2O2ŗĶH2O B£®NaŗĶO2
C£®CuOŗĶC D£®CŗĶO2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ³£ÓƵÄøÉŌļ¼ĮÖŠ£¬²»ÄÜÓĆĄ“øÉŌļCl2µÄŹĒ(””””)
A£®ÅØĮņĖį B£®¼īŹÆ»Ņ
C£®ĪŽĖ®ĀČ»ÆøĘ D£®P2O5
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ä³Ń§ÉśÓ¦ÓĆĻĀĶ¼ĖłŹ¾µÄ·½·ØŃŠ¾æĪļÖŹµÄŠŌÖŹ£¬ĘäÖŠĘųĢåAµÄÖ÷ŅŖ³É·ÖŹĒĀČĘų£¬ŌÓÖŹŹĒæÕĘųŗĶĖ®ÕōĘų”£»Ų“šĻĀĮŠĪŹĢā£ŗ
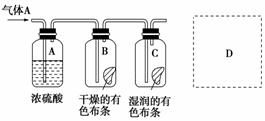
(1)øĆĻīŃŠ¾æ(ŹµŃé)µÄÖ÷ŅŖÄæµÄŹĒ________________________________”£
(2)ÅØĮņĖįµÄ×÷ÓĆŹĒ________£¬ÓėŃŠ¾æÄæµÄÖ±½ÓĻą¹ŲµÄŹµŃéĻÖĻóŹĒ
_____________________________________________________________
_____________________________________________________________ӣ
(3)“ÓĪļÖŹŠŌÖŹµÄ·½ĆęĄ“æ“£¬ÕāŃłµÄŹµŃéÉč¼Ę»¹“ęŌŚŹĀ¹ŹŅž»¼£¬ŌŅņŹĒ
______________________________________________________________
_____________________________________________________________ӣ
ĒėŌŚĶ¼ÖŠD“¦ŅŌĶ¼µÄŠĪŹ½±ķĆ÷æĖ·žŹĀ¹ŹŅž»¼µÄ“ėŹ©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŖĖŲÖÜĘŚ±ķÖŠīéŌŖĖŲµÄŹż¾Ż¼ūÓŅĶ¼£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
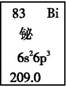
A.BiŌŖĖŲµÄÖŹĮæŹżŹĒ209.0 B.BiŌŖĖŲÖŠ×ÓŹżĪŖ83
C.BiŌ×Ó6pŃĒ²ćÓŠÓŠŅ»øöĪ“³É¶Ōµē×Ó D.BiĪ»ÓŚµŚĮłÖÜĘŚVA×壬ŹōÓŚpĒų
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®ĢĒĄą”¢ÓĶÖ¬”¢µ°°×ÖŹ¶¼ÄÜ·¢ÉśĖ®½ā·“Ó¦ B£®ĢĒĄą”¢ÓĶÖ¬”¢µ°°×ÖŹ¶¼ŹĒÓÉC”¢H”¢OČżÖÖŌŖĖŲ×é³ÉµÄ
C£®ĢĒĄą”¢ÓĶÖ¬”¢µ°°×ÖŹ¶¼ŹĒøß·Ö×Ó»ÆŗĻĪļ D£®ÓĶÖ¬ÓŠÓĶŗĶÖ¬·¾Ö®·Ö£¬µ«¶¼ŹōÓŚõ„
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com