
 ĻĀĮŠĮ½ÖÖ·½°øÖʱøĒāŃõ»ÆĀĮ£ŗ
ĻĀĮŠĮ½ÖÖ·½°øÖʱøĒāŃõ»ÆĀĮ£ŗ| A£® | ŌŚaĒśĻß±ķŹ¾µÄŹĒĻņYČÜŅŗÖŠ¼ÓČėĻ”ŃĪĖį | |
| B£® | ·½°ø¢ņ±Č·½°ø¢ńÉś³Éøü¶ąµÄĘųĢå | |
| C£® | ŌŚMµćŹ±£¬Į½·½°øÖŠĖłµĆČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹżĻąĶ¬ | |
| D£® | MµćŅŌŗó£¬a”¢bĮ½ĢõĒśĻß½«ÖŲŗĻĪŖŅ»Ģõ |
·ÖĪö ·½°øI»į·¢Éś·“Ó¦£ŗ2Al+6HCl=2AlCl3+3H2”ü”¢AlCl3+3NaOH=Al£ØOH£©3”ż+3NaCl£»
·½°ø¢ņ»į·¢Éś·“Ó¦£ŗ2Al+2NaOH+2H2OØT2NaAlO2+3H2”ü”¢NaAlO2+HCl+H2=Al£ØOH£©3”ż+NaCl£»
ĀĮµÄĪļÖŹµÄĮæ=$\frac{2.7g}{27g/mol}$=0.1mol£¬n£ØHCl£©=n£ØNaOH£©=3mol•L-1”Į0.1L=0.3mol£¬øł¾Ż·“Ó¦·½³ĢŹ½£¬æÉÖŖ·½°øIÖŠAlÓėHClĒ”ŗĆ·“Ó¦£¬·½°ø¢ņÖŠNaOHÓŠŹ£Óą£®
A£®·½°øIÖŠŌŁµĪ¼ÓNaOH£¬Į¢¼“Éś³É³Įµķ£¬·½°ø¢ņÖŠŌŁ¼ÓČėHCl£¬ĻČÖŠŗĶŹ£ÓąµÄNaOH£¬ŌŁ·“Ӧɜ³ÉĒāŃõ»ÆĀĮ³Įµķ£»
B£®øł¾Ż·½³ĢŹ½¼ĘĖć½ųŠŠ¼ĘĖć£»
C£®Mµć×īÖÕ¾łĪŖNaClČÜŅŗ£¬ČÜŅŗĢå»ż²»±ä£¬Éś³ÉNaClĻąµČ£»
D£®·¢Éś·“Ó¦£ŗAl£ØOH£©3+3HCl=AlCl3+3H2O£¬Al£ØOH£©3+NaOHØTNaAlO2+2H2O£¬ĒāŃõ»ÆĀĮČܽāĻūŗÄŃĪĖįÓėĒāŃõ»ÆÄĘČÜŅŗĢå»ż²»ĻąµČ£®
½ā“š ½ā£ŗ·½°øI»į·¢Éś·“Ó¦£ŗ2Al+6HCl=2AlCl3+3H2”ü”¢AlCl3+3NaOH=Al£ØOH£©3”ż+3NaCl£»
·½°ø¢ņ»į·¢Éś·“Ó¦£ŗ2Al+2NaOH+2H2OØT2NaAlO2+3H2”ü”¢NaAlO2+HCl+H2=Al£ØOH£©3”ż+NaCl£»
ĀĮµÄĪļÖŹµÄĮæ=$\frac{2.7g}{27g/mol}$=0.1mol£¬n£ØHCl£©=n£ØNaOH£©=3mol•L-1”Į0.1L=0.3mol£¬øł¾Ż·“Ó¦·½³ĢŹ½£¬æÉÖŖ·½°øIÖŠAlÓėHClĒ”ŗĆ·“Ó¦£¬·½°ø¢ņÖŠNaOHÓŠŹ£Óą£®
A£®·½°øIÖŠŌŁµĪ¼ÓNaOH£¬Į¢¼“Éś³É³Įµķ£¬·½°ø¢ņÖŠŌŁ¼ÓČėHCl£¬ĻČÖŠŗĶŹ£ÓąµÄNaOH£¬ŌŁ·“Ӧɜ³ÉĒāŃõ»ÆĀĮ³Įµķ£¬¹ŹĖłŅŌaĒśĻß±ķŹ¾µÄŹĒĻņXČÜŅŗÖŠ¼ÓČėNaOHČÜŅŗ£¬¹ŹA“ķĪó£»
B£®AlĶźČ«·“Ó¦£¬øł¾Ż·½³ĢŹ½æÉÖŖÉś³ÉĒāĘųµÄĢå»żĻąµČ£¬¹ŹB“ķĪó£»
C£®Mµć×īÖÕ¾łĪŖNaClČÜŅŗ£¬ČÜŅŗĢå»ż²»±ä£¬Éś³ÉNaClĻąµČ£¬¹ŹŌŚMµćŹ±£¬Į½·½°øÖŠĖłµĆČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹżĻąĶ¬£¬¹ŹCÕżČ·£»
D£®·¢Éś·“Ó¦£ŗAl£ØOH£©3+3HCl=AlCl3+3H2O£¬Al£ØOH£©3+NaOHØTNaAlO2+2H2O£¬ĒāŃõ»ÆĀĮČܽāĻūŗÄŃĪĖįÓėĒāŃõ»ÆÄĘČÜŅŗĢå»ż²»ĻąµČ£¬MµćŅŌŗó£¬a”¢bĮ½ĢõĒśĻß²»ÄÜÖŲŗĻĪŖŅ»Ģõ£¬¹ŹD“ķĪó£¬
¹ŹŃ”C£®
µćĘĄ ±¾ĢāŅŅĒāŃõ»ÆĀĮÖʱø·½°øĪŖŌŲĢ壬漲é»Æѧ·½³ĢŹ½¼ĘĖć”¢»Æѧ·“Ó¦Ķ¼ĻóĪŹĢāµČ£¬Ć÷Č··¢ÉśµÄ·“Ó¦ŹĒ½āĢā¹Ų¼ü£¬ÄѶČÖŠµČ£®


 ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ijŠ£æĪĶāŹµŃ銔×éĶ¬Ń§Éč¼ĘĮĖČēĶ¼×°ÖĆÖĘČ”²¢ŹÕ¼ÆŅ»ĘæĘųĢ壬Ēė°ļÖśøĆŠ”×éĶ¬Ń§Ķź³ÉĻĀ±ķ£®
ijŠ£æĪĶāŹµŃ銔×éĶ¬Ń§Éč¼ĘĮĖČēĶ¼×°ÖĆÖĘČ”²¢ŹÕ¼ÆŅ»ĘæĘųĢ壬Ēė°ļÖśøĆŠ”×éĶ¬Ń§Ķź³ÉĻĀ±ķ£®| ÖĘČ”µÄĘųĢå | Ņ© Ę· | »Æѧ·½³ĢŹ½ |
| O2 | H2O2ӢMnO2 | 2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2ӟ |
| H2 | ŅŅ“¼”¢Na | 2CH2CH2OH+2Na=2CH3CH2ONa+H2”ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĪĀ¶ČŅ»¶ØŹ±£¬µ±ČÜŅŗÖŠc£ØBa2+£©”Įc£ØSO42-£©=1.07”Į10-10Ź±£¬“ĖČÜŅŗĪŖBaSO4µÄ±„ŗĶČÜŅŗ | |
| B£® | ŅņĪŖKsp£ØBaCO3£©£¾Ksp£ØBaSO4£©£¬ĖłŅŌĪŽ·Ø½«BaSO4×Ŗ»ÆĪŖBaCO3 | |
| C£® | 25”ꏱ£¬ŌŚĪ“ČܽāĶźBaCO3µÄ±„ŗĶČÜŅŗÖŠµĪČėÉŁĮæNa2SO4ČÜŅŗŗóÓŠBaSO4³ĮµķĪö³ö£¬“ĖŹ±ČÜŅŗÖŠc£ØCO32-£©£ŗc£ØSO42-£©=24.11 | |
| D£® | ŌŚ±„ŗĶBaCO3ČÜŅŗÖŠ¼ÓČėÉŁĮæNa2CO3¹ĢĢ壬æÉŹ¹c£ØBa2+£©¼õŠ”£¬BaCO3µÄČܶȻż²»±ä |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Fe2O3³Źŗģ×ŲÉ«£¬æÉÓĆ×öŗģÉ«ŃÕĮĻ | |
| B£® | ÅØĮņĖįÄÜøÆŹ“½šŹō£¬²»ÄÜÓĆČĪŗĪ½šŹōČŻĘ÷Ź¢×°ÅØĮņĖį | |
| C£® | ĀČĘųÓŠ¶¾£¬ĄūÓĆÕāŅ»ŠŌÖŹæɽųŠŠ×ŌĄ“Ė®µÄɱ¾śĻū¶¾ | |
| D£® | Ć¾”¢ĀĮ¶¼±Č½ĻČķ£¬²»ÄÜÓĆ×ö×°ŹĪ²ÄĮĻ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓƶčŠŌµē¼«µē½āCuCl2ČÜŅŗ£¬Ņõ¼«Īö³ö16gĶŹ±£¬ĻßĀ·ÖŠĶعżµÄµē×ÓŹżĪŖNA | |
| B£® | ±ź×¼×“æöĻĀ£¬2.24LŅŅČ©ĶźČ«Č¼ÉÕĖłµĆCO2·Ö×ÓŹżŌ¼ĪŖ0.2NA | |
| C£® | ±ź×¼×“æöĻĀ£¬22.4LÄŹĘųÖŠŗ¬ÓŠNAøöÄŹŌ×Ó | |
| D£® | 1mol¼×»łĖłŗ¬µē×ÓŹżŌ¼ĪŖ8NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±½·Ö×ÓÖŠ²»“ęŌŚĢ¼Ģ¼Ė«¼ü£¬ĖłŅŌ²»ÄÜ·¢Éś¼Ó³É·“Ó¦ | |
| B£® | ŅŅ“¼ÄÜ·¢ÉśŃõ»Æ·“Ó¦£¬ŅŅĖįŌņ²»æÉÄÜ·¢ÉśŃõ»Æ·“Ó¦ | |
| C£® | ŅŅĖįŗĶŅŅ“¼ÖĘŅŅĖįŅŅõ„Óė±½¼×ĖįŅŅõ„Ė®½āÖʱ½¼×ĖįŗĶŅŅ“¼¶¼ŹōÓŚČ”“ś·“Ó¦ | |
| D£® | ¼×±½ÓėĀČĘųŌŚ¹āÕÕĻĀ·“Ó¦Ö÷ŅŖÉś³É2£¬4-¶žĀČ¼×±½ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1mol${\;}_{8}^{16}$OD- ÖŠŗ¬ÓŠµÄÖŹ×ÓŹż”¢ÖŠ×ÓŹż¾łĪŖ9NA | |
| B£® | 3.6 gŹÆÄ«ŗĶC60µÄ»ģŗĻĪļÖŠ£¬ŗ¬ÓŠµÄĢ¼Ō×ÓŹżĪŖ0.3NA | |
| C£® | ·“Ó¦3H2£Øg£©+N2£Øg£©?2NH3£Øg£©”÷H=-92kJ/mol·Å³öČČĮæ9.2kJŹ±£¬×ŖŅʵē×Ó0.6NA | |
| D£® | ±ź×¼×“æöĻĀ£¬4.48LĪģĶéŗ¬ÓŠµÄ·Ö×ÓŹżĪŖ0.2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆĮņĖįĒåĻ“¹ųĀÆÖŠµÄĖ®¹ø | |
| B£® | ČÜŅŗŹĒµēÖŠŠŌµÄ£¬½ŗĢåŹĒ“ųµēµÄ | |
| C£® | ĄūÓƶ”“ļ¶ūŠ§Ó¦æÉŅŌĒų±šČÜŅŗÓė½ŗĢå | |
| D£® | ÉÕ¼ī”¢±ł“×Ėį”¢ĖÄĀČ»ÆĢ¼¾łĪŖµē½āÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
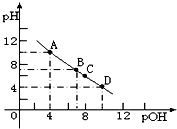 ³£ĪĀŹ±£¬½«°±ĘųČÜÓŚĖ®ŠĪ³ÉŅ»ÅضČĪŖ0.1mol/L£¬pHĪŖ10µÄ°±Ė®ČÜŅŗ100mL£®
³£ĪĀŹ±£¬½«°±ĘųČÜÓŚĖ®ŠĪ³ÉŅ»ÅضČĪŖ0.1mol/L£¬pHĪŖ10µÄ°±Ė®ČÜŅŗ100mL£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com