
ČĖĆĒŅŃ¾ŃŠÖĘ³öŅŌ±ūĶéĪŖČ¼ĮĻµÄŠĀŠĶČ¼ĮĻµē³Ų£¬µē½āÖŹĪŖČŪČŚĢ¼ĖįŃĪ£¬µē³Ų×Ü·“Ó¦·½³ĢŹ½ĪŖ£ŗC3H8+5O2=3CO2+4H2O”£
£Ø1£©ŅŃÖŖ£ŗ2C3H8£Øg£©+7O2£Øg£©=6CO£Øg£©+8H2O£Øl£©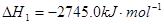
C£Øs£©+O2£Øg£©=CO2£Øg£© 
2C£Øs£©+O2£Øg£©=2CO£Øg£© 
Ōņ·“Ó¦C3H8£Øg£©+5O2£Øg£©=3CO2£Øg£©+4H2O£Ø1£©µÄ”÷H___________________”£.
£Ø2£©øƵē³ŲµÄÕż¼«ĶØČėO2ŗĶCO2£¬øŗ¼«ĶØČė±ūĶ飬ŌņÕż¼«µÄµē¼«·“Ó¦Ź½ĪŖ_________________£¬µē³Ų¹¤×÷Ź±CO32”ŖŅĘĻņ_____________¼«”£
£Ø3£©ÓĆøƵē³Ųµē½ā1L 1 mol”¤L”Ŗ1µÄAgNO3ČÜŅŗ£¬“Ėµē½ā³Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________________£»µ±øƵē³ŲĻūŗÄ0.005molC3H8Ź±£¬ĖłµĆČÜŅŗµÄpHĪŖ__________£ØČÜŅŗĢå»ż±ä»ÆŗöĀŌ²»¼Ę£©
£Ø1£©”Ŗ2221.5kJ”¤mol”Ŗ1£Ø2·Ö£¬ĪŽµ„Ī»»ņµ„Ī»Š““ķ²»øų·Ö£©
£Ø2£©O2+2CO2+4e”Ŗ =2CO32”Ŗ£Ø2·Ö£©øŗ£Ø1·Ö£©
£Ø3£©4AgNO3+2H2O 4Ag+O2”ü +4HNO3£Ø2·Ö£¬²»Š“·“Ó¦Ģõ¼ž»ņ²»ÅäĘ½²»øų·Ö£© 1£Ø2·Ö£©
4Ag+O2”ü +4HNO3£Ø2·Ö£¬²»Š“·“Ó¦Ģõ¼ž»ņ²»ÅäĘ½²»øų·Ö£© 1£Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©øł¾Ż·½³ĢŹ½ĻČŗóĖ³ŠņÉč·Ö±šĪŖ¢Ł”¢¢Ś”¢¢Ū£¬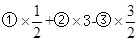 £¬µĆµ½·½³ĢŹ½C3H8£Øg£©+5O2£Øg£©=3CO2£Øg£©+4H2O£Ø1£©£»·“Ó¦ČČŅ²°“ÕÕÉĻŹ½¼ĘĖć”÷H=
£¬µĆµ½·½³ĢŹ½C3H8£Øg£©+5O2£Øg£©=3CO2£Øg£©+4H2O£Ø1£©£»·“Ó¦ČČŅ²°“ÕÕÉĻŹ½¼ĘĖć”÷H= =”Ŗ2221.5kJ”¤mol”Ŗ1”£
=”Ŗ2221.5kJ”¤mol”Ŗ1”£
£Ø2£©ŃõĘųŌŚÕż¼«·“Ó¦£¬O2+2CO2+4e”Ŗ=2CO32”Ŗ£¬Ōµē³ŲÖŠŅõĄė×ÓĻņøŗ¼«ŅĘ¶Æ£»
£Ø3£©µē½āĻõĖįŅų£¬ŹĒ·ÅŃõÉśĖįŠĶ£¬4AgNO3+2H2O 4Ag+O2”ü +4HNO3£»µ±øƵē³ŲĻūŗÄ0.005molC3H8Ź±£¬×ŖŅʵē×ÓŹĒ0.1mol£¬Éś³É0.1molH+£¬ĒāĄė×ÓÅضČĪŖ0.1mol/L£¬pHĪŖ1”£
4Ag+O2”ü +4HNO3£»µ±øƵē³ŲĻūŗÄ0.005molC3H8Ź±£¬×ŖŅʵē×ÓŹĒ0.1mol£¬Éś³É0.1molH+£¬ĒāĄė×ÓÅضČĪŖ0.1mol/L£¬pHĪŖ1”£
æ¼µć£ŗæ¼²éČČ»Æѧ·½³ĢŹ½µÄŹéŠ“¼°¼ĘĖć£¬Ōµē³ŲÓėµē½ā³ŲµÄŌĄķ¼°¼ĘĖć”£øĒĖ¹¶ØĀÉŅŖĒó·½³ĢŹ½æÉŅŌĻą¼Ó¼õ£¬·“Ó¦ČČŅ²æÉŅŌĻą¼Ó¼õ”£ŹōÓŚ½Ļ×ŪŗĻĢā”£


 »Ŗ¶«Ź¦“ó°ęŅ»æĪŅ»Į·ĻµĮŠ“š°ø
»Ŗ¶«Ź¦“ó°ęŅ»æĪŅ»Į·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
µŖŹĒµŲĒņÉĻŗ¬Įæ·įø»µÄŅ»ÖÖŌŖĖŲ£¬Ę䵄֏¼°»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²ś”¢Éś»īÖŠÓŠ×ÅÖŲŅŖ×÷ÓĆ”£
£Ø1£©Ņ»¶ØĪĀ¶ČĻĀ£¬ŌŚ1LČŻ»żŗć¶ØµÄĆܱÕČŻĘ÷ÖŠ³äČė2 mol N2ŗĶ8molH2²¢·¢Éś·“Ó¦”£10min“ļĘ½ŗā£¬²āµĆ°±ĘųµÄÅضČĪŖ0£®4 mol”¤L£1£¬“ĖŹ±µŖĘųµÄ×Ŗ»ÆĀŹĪŖ________”£ČōĻėĢįøß°±ĘųµÄ²śĀŹ£¬øł¾Ż»ÆŃ§Ę½ŗāŅʶÆŌĄķ£¬Ģį³öŗĻĄķµÄ½ØŅé______________£ØŠ“³öŅ»Ģõ¼“æÉ£©”£
£Ø2£©ČēĶ¼ŹĒ1mol NO2£Øg£©ŗĶ1mol CO£Øg£©·“Ӧɜ³Élmol CO2£Øg£©ŗĶ1 mol NO£Øg£©¹ż³ĢÖŠÄÜĮæ±ä»ÆŹ¾ŅāĶ¼£¬ĒėŠ“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½_____________________”£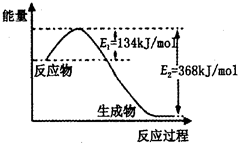
£Ø3£©ŌŚČŻ»żŗć¶ØµÄĆܱÕČŻĘ÷ÖŠ£¬½ųŠŠČēĻĀ·“Ó¦£ŗN2£Øg£©£«3H2£Øg£© 2NH3£Øg£© ”÷H£¼0£¬ĘäĘ½ŗā³£ŹżKÓėĪĀ¶ČTµÄ¹ŲĻµČēĻĀ±ķ£ŗ
2NH3£Øg£© ”÷H£¼0£¬ĘäĘ½ŗā³£ŹżKÓėĪĀ¶ČTµÄ¹ŲĻµČēĻĀ±ķ£ŗ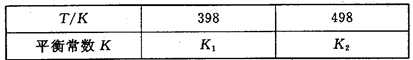
¢ŁøĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½£ŗK£½_____________£»
¢ŚŹŌÅŠ¶ĻK1__________K2£ØĢīŠ“”°£¾”±”°£½”±»ņ”°£¼”±£½£»
¢ŪNH3£Øg£©Č¼Éյķ½³ĢŹ½ĪŖ£ŗ4NH3£Øg£©£«7O2£Øg£©£½4NO2£Øg£©£«6H2O£Øl£©£¬ŅŃÖŖ£ŗ
H2£Øg£©£«O2£Øg£© 2H2O£Øl£© ”÷H£½£483£®6 kJ£Æmol
2H2O£Øl£© ”÷H£½£483£®6 kJ£Æmol
N2£Øg£©£«2O2£Øg£© 2NO2£Øg£© ”÷H£½£«67£®8 kJ£Æmol
2NO2£Øg£© ”÷H£½£«67£®8 kJ£Æmol
N2£Øg£©£«3H2£Øg£© 2NH3£Øg£© ”÷H£½£92£®0 kJ£Æmol
2NH3£Øg£© ”÷H£½£92£®0 kJ£Æmol
Ēė¼ĘĖćNH3£Øg£©µÄČ¼ÉÕČČ________kJ£Æmol”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĦĶŠĀŽĄ¹«Ė¾æŖ·¢ĮĖŅ»ÖÖŅŌ¼×“¼ĪŖŌĮĻ£¬ŅŌKOHĪŖµē½āÖŹµÄÓĆÓŚŹÖ»śµÄæɳäµēµÄøߊ§Č¼ĮĻµē³Ų£¬³äŅ»“ĪµēæÉŅŌĮ¬ŠųŹ¹ÓĆŅ»øöŌĀ”£ŅŃÖŖøƵē³ŲµÄ×Ü·“Ó¦Ź½ĪŖ£ŗ
2CH3OH£«3O2£«4KOH 2K2CO3£«6H2O
2K2CO3£«6H2O
ĒėĢīæÕ£ŗ
(1)·ÅµēŹ±£ŗøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ_________________________________________________________”£
(2)ĶØČė¼×“¼Ņ»¶ĖµÄµē¼«ŹĒ________¼«£¬µē³ŲŌŚ·Åµē¹ż³ĢÖŠČÜŅŗµÄpH½«_______ (Ģī”°ÉĻÉż”±”¢”°ĻĀ½µ”±»ņ”°²»±ä”±)”£
(3)ČōŌŚ³£ĪĀ”¢³£Ń¹ĻĀ£¬1 g CH3OHČ¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®Ź±·Å³ö22.68 kJµÄČČĮ棬±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
µŖŹĒµŲĒņÉĻŗ¬Įæ·įø»µÄŅ»ÖÖŌŖĖŲ£¬µŖ¼°Ęä»ÆŗĻĪļŌŚ¹¤ Å©ŅµÉś²ś”¢Éś»īÖŠÓŠ×ÅÖŲŅŖ×÷ÓĆ£¬ 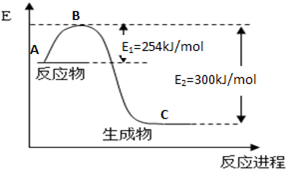
£Ø1£©ÉĻĶ¼ŹĒN2(g)ŗĶH2(g)·“Ӧɜ³É1mol NH3(g)¹ż³ĢÖŠÄÜĮæ±ä»ÆŹ¾ŅāĶ¼£¬ĒėŠ“³öN2ŗĶH2·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø2£©ČōŅŃÖŖĻĀĮŠŹż¾Ż£ŗ
| »Æѧ¼ü | H£H | N”ŌN |
| ¼üÄÜ/kJ”¤mol£1 | 435 | 943 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ŅŃÖŖŗģĮױȰ×Į×ĪČ¶Ø£¬ÓÖÖŖ£ŗ4P(°×Į×£¬s£©+5O2£Øg£©£½2P2O5£Øs£© ”÷H1£»
4P£ØŗģĮ×£¬s£©+5O2£Øg£©£½2P2O5£Øs£© ”÷H2£¬Ōņ¦¤H1ŗĶ¦¤H2µÄ¹ŲĻµŹĒ”÷H1 ”÷H2£ØĢī”°£¾”±”¢”°£¼”±
»ņ”°£½”±£©”£
£Ø2£©ŅŃÖŖH2£Øg£©ŗĶCH3OH£Øl£©µÄČ¼ÉÕČČ”÷H·Ö±šĪŖ-285.8kJ”¤mol-1ŗĶ-726.5kJ”¤mol-1£¬Š“³öÓÉCO2
ŗĶH2Éś³ÉŅŗĢ¬¼×“¼ŗĶŅŗĢ¬Ė®µÄČČ»Æѧ·½³ĢŹ½ ”£
£Ø3£©ŅŃÖŖŅ»¶ØĪĀ¶ČĻĀ£¬ĻĀĮŠ·“Ó¦µÄĘ½ŗā³£Źż£ŗSO2(g)+1/2O2(g)  SO3(g) K1,CO(g)+1/2O2(g)
SO3(g) K1,CO(g)+1/2O2(g)  CO2(g) K2”£ŌņĻąĶ¬ĪĀ¶ČĻĀ·“Ó¦SO2(g)+CO2(g)
CO2(g) K2”£ŌņĻąĶ¬ĪĀ¶ČĻĀ·“Ó¦SO2(g)+CO2(g)  SO3(g)+CO(g)µÄĘ½ŗā³£ŹżĪŖ ”£
SO3(g)+CO(g)µÄĘ½ŗā³£ŹżĪŖ ”£
£ØÓĆK1”¢K2±ķŹ¾£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÕÓĘų£ØÖ÷ŅŖ³É·ÖŹĒ¼×Ķ飩ŹĒŅ»ÖÖĮ®¼ŪµÄÄÜŌ“£¬°ŃÅ©“åÖŠ“óĮæ“ęŌŚµÄÅ©×÷Īļ½ÕøŃ”¢ŌÓ²Ż”¢ČĖŠó·ą±ćµČŌŚÕÓĘų³ŲÖŠ·¢½Ķ£¬±ćæɲśÉśÕÓĘų£¬ÕÓĘųĶźČ«Č¼ÉÕæÉŅŌÓĆĄ“µćµĘ”¢×ö·¹”£
£Ø1£©ŌŚ101kPaŹ±£¬32gCH4Č¼ÉÕÉś³ÉCO2ŗĶĘųĢ¬H2O£¬·Å³ö1604kJµÄČČĮ棬Š“³ö¼×ĶéČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ__________________________________________________________”£
£Ø2£©120”ę£¬Č”CH4ŗĶ¹żĮæO2µÄ»ģŗĻĘųĢå¹²0.4mol£¬µćČ¼Ź¹Ęä³ä·Ö·“Ó¦£¬½«Č¼ÉÕŗóµÄĘųĢåĶعż×ćĮæµÄ¼īŹÆ»Ņ£ØNaOHŗĶCaOµÄ¹ĢĢå»ģŗĻĪļ£©³ä·ÖĪüŹÕ£¬¼īŹÆ»ŅŌöÖŲ8g”£¼ĘĖć£ŗŌ»ģŗĻĘųĢåÖŠCH4ŗĶO2µÄĢå»ż±ČŹĒ¶ąÉŁ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶ž¼×ĆŃŹĒ”ŖÖÖÖŲŅŖµÄĒå½ąČ¼ĮĻ£¬Ņ²æÉĢę“ś·śĄū°ŗ×÷ÖĘĄä¼ĮµČ£¬¶Ō³ōŃõ²ćĪŽĘĘ»µ×÷ÓĆ”£¹¤ŅµÉĻæÉĄūÓĆĆŗµÄĘų»Æ²śĪļ£ØĖ®ĆŗĘų£©ŗĻ³É¶ž¼×ĆŃ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĆŗµÄĘų»ÆµÄÖ÷ŅŖ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ_______________________________________”£
£Ø2£©ĆŗµÄĘų»Æ¹ż³ĢÖŠ²śÉśµÄÓŠŗ¦ĘųĢåÓĆČÜŅŗĪüŹÕ£¬Éś³ÉĮ½ÖÖĖįŹ½ŃĪ£¬øĆ·“Ó¦µÄ
»Æѧ·½³ĢŹ½ĪŖ__________________________________________________________”£
£Ø3£©ĄūÓĆĖ®ĆŗĘųŗĻ³É¶ž¼×ĆѵÄČż²½·“Ó¦ČēĻĀ£ŗ
¢Ł2H2£Øg£©+CO£Øg£© CH3OH£Øg£©£»”÷H=-90.8kJ”¤mol£1
CH3OH£Øg£©£»”÷H=-90.8kJ”¤mol£1
¢Ś2CH3OH£Øg£© CH3OCH3£Øg£©+H2O£Øg£©£»”÷H=-23.5kJ”¤mol£1
CH3OCH3£Øg£©+H2O£Øg£©£»”÷H=-23.5kJ”¤mol£1
¢ŪCO£Øg£©+H2O£Øg£© CO2£Øg£©+H2£Øg£©£»”÷H=-41.3kJ”¤mol£1
CO2£Øg£©+H2£Øg£©£»”÷H=-41.3kJ”¤mol£1
×Ü·“Ó¦£ŗ3H2£Øg£©+3CO£Øg£© CH3OCH3£Øg£©+CO2£Øg£©µÄ”÷H= £»
CH3OCH3£Øg£©+CO2£Øg£©µÄ”÷H= £»
Ņ»¶ØĢõ¼žĻĀµÄĆܱÕČŻĘ÷ÖŠ£¬øĆ×Ü·“Ó¦“ļµ½Ę½ŗā£¬ŅŖĢįøßCOµÄ×Ŗ»ÆĀŹ£¬æÉŅŌ²ÉČ”µÄ“ėŹ©ŹĒ
________________£ØĢī×ÖÄø“śŗÅ£©”£
a£®øßĪĀb£®¼ÓČė“߻ƼĮc£®¼õÉŁCO2µÄÅضČd£®Ōö¼ÓCOµÄÅضČe£®·ÖĄė³ö¶ž¼×ĆŃ
£Ø4£©ŅŃÖŖ·“Ó¦¢Ś2CH3OH£Øg£© CH3OCH3£Øg£©+H2O(g)ijĪĀ¶ČĻĀµÄĘ½ŗā³£ŹżĪŖ400”£
CH3OCH3£Øg£©+H2O(g)ijĪĀ¶ČĻĀµÄĘ½ŗā³£ŹżĪŖ400”£
“ĖĪĀ¶ČĻĀ£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČėCH3OH£¬·“Ó¦µ½Ä³Ź±æĢ²āµĆø÷×é·ÖµÄÅضČČēĻĀ£ŗ
| ĪļÖŹ | CH3OH | CH3OCH3 | H2O |
| ÅضČ/£Ømol?L£© | 0.44 | 0.6 | 0.6 |
 _______
_______ £ØĢī”°>”±”¢”°<”±»ņ”°=”±£©”£
£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ĒāĘųŹĒŅ»ÖÖĒå½ąÄÜŌ“£¬ĒāĘųµÄÖĘČ”Óė“¢“ęŹĒĒāÄÜŌ“ĄūÓĆĮģÓņµÄŃŠ¾æČČµć”£
£Ø1£©ŅŌ¼×ĶéĪŖŌĮĻÖĘČ”ĒāĘųŹĒ¹¤ŅµÉĻ³£ÓƵÄÖĘĒā·½·Ø”£ŅŃÖŖ£ŗ
CH4(g)£«H2O(g) ===CO(g)£«3H2(g) ¦¤H£½+206.2 kJ/mol
CH4(g)£«CO2(g) ===2CO(g)£«2H2(g) ¦¤H£½+247.4 kJ/mol
CH4(g)ÓėH2O(g)·“Ӧɜ³ÉCO2(g)ŗĶH2(g)µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ĮņĢśæó(FeS2)Č¼ÉÕ²śÉśµÄSO2ĶعżĻĀĮŠµāŃ»·¹¤ŅÕ¹ż³Ģ¼ČÄÜÖĘH2SO4£¬ÓÖÄÜÖĘH2”£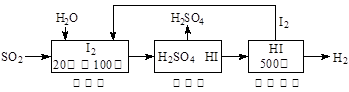
ŅŃÖŖ1g FeS2ĶźČ«Č¼Éշųö7.1 kJČČĮ棬FeS2Č¼ÉÕ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
øĆŃ»·¹¤ŅÕ¹ż³ĢµÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø3£©µē½āÄņĖŲ[CO(NH2)2]µÄ¼īŠŌČÜŅŗÖĘĒāµÄ×°ÖĆŹ¾ŅāĶ¼¼ūĶ¼£Øµē½ā³ŲÖŠøōĤ½ö×čÖ¹ĘųĢåĶعż£¬Ņõ”¢Ńō¼«¾łĪŖ¶čŠŌµē¼«£©”£µē½āŹ±£¬Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£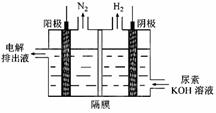
£Ø4£©ÓĆĪüŹÕH2ŗóµÄĻ”ĶĮ“¢ĒāŗĻ½š×÷ĪŖµē³Ųøŗ¼«²ÄĮĻ(ÓĆMH±ķŹ¾)£¬NiO(OH)×÷ĪŖµē³ŲÕż¼«²ÄĮĻ£¬KOHČÜŅŗ×÷ĪŖµē½āÖŹČÜŅŗ£¬æÉÖʵĆøßČŻĮ棬³¤ŹŁĆüµÄÄųĒāµē³Ų”£µē³Ų³ä·ÅµēŹ±µÄ×Ü·“Ó¦ĪŖ£ŗ
NiO(OH)£«MH Ni(OH)2£«M
Ni(OH)2£«M
¢Łµē³Ų·ÅµēŹ±£¬Õż¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
¢Ś³äµēĶź³ÉŹ±£¬Ni(OH)2Č«²æ×Ŗ»ÆĪŖNiO(OH)”£Čō¼ĢŠų³äµē½«ŌŚŅ»øöµē¼«²śÉśO2£¬O2Ą©É¢µ½ĮķŅ»øöµē¼«·¢Éśµē¼«·“Ó¦±»ĻūŗÄ£¬“Ó¶ų±ÜĆā²śÉśµÄĘųĢåŅżĘšµē³Ų±¬ÕØ£¬“ĖŹ±£¬Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø5£©Mg2CuŹĒŅ»ÖÖ“¢ĒāŗĻ½š”£350”ꏱ£¬Mg2CuÓėH2·“Ó¦£¬Éś³ÉMgCu2ŗĶ½öŗ¬Ņ»ÖÖ½šŹōŌŖĖŲµÄĒā»ÆĪļ£ØĘäÖŠĒāµÄÖŹĮæ·ÖŹżĪŖ0.077£©”£Mg2CuÓėH2·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹¤ŅµŗĻ³É°±ÓėÖʱøĻõĖįŅ»°ćæÉĮ¬ŠųÉś²ś£¬Į÷³ĢČēĻĀ£ŗ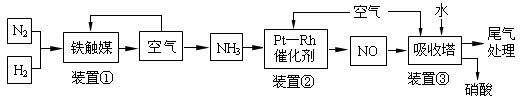
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Ä³æĘŃŠŠ”×éŃŠ¾æ£ŗŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬øıäĘšŹ¼ĪļĒāĘųµÄĪļÖŹµÄĮ棬¶Ō·“Ó¦N2(g)£«3H2(g) 2NH3(g) ¦¤H£½£92.4 kJ”¤mol-1µÄÓ°Ļģ”£ŹµŃé½į¹ūČēĶ¼ĖłŹ¾£ŗ£ØĶ¼ÖŠT±ķŹ¾ĪĀ¶Č£¬n±ķŹ¾ĪļÖŹµÄĮ棩
2NH3(g) ¦¤H£½£92.4 kJ”¤mol-1µÄÓ°Ļģ”£ŹµŃé½į¹ūČēĶ¼ĖłŹ¾£ŗ£ØĶ¼ÖŠT±ķŹ¾ĪĀ¶Č£¬n±ķŹ¾ĪļÖŹµÄĮ棩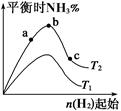
¢ŁĶ¼ĻńÖŠT2ŗĶT1µÄ¹ŲĻµŹĒ£ŗT2 T1(Ģī”°>”±”¢”°<”±”¢”°=”±»ņ”°ĪŽ·ØČ·¶Ø”±)”£
¢Ś±Č½ĻŌŚa”¢b”¢cČżµćĖł“¦µÄĘ½ŗāדĢ¬ÖŠ£¬N2µÄ×Ŗ»ÆĀŹ×īøߵďĒ (Ģī×ÖÄø)”£
¢ŪŅŖŹ¹·“Ó¦ŗ󰱵İŁ·Öŗ¬Įæ×ī“ó£¬ŌņŌŚĘšŹ¼ĢåĻµÖŠŌĮĻĶ¶ĮĻ±Čn(H2)/n(N2) 3£ØĢī ”°>”±”¢”°<”±”¢”°=”±»ņ”°ĪŽ·ØČ·¶Ø”±£©”£ČōČŻĘ÷ČŻ»żŗćĪŖ1 L£¬ĘšŹ¼×“Ģ¬n(H2)="3" mol£¬·“Ó¦“ļµ½Ę½ŗāŹ±H2µÄ×Ŗ»ÆĀŹĪŖ60%£¬Ōņ“ĖĢõ¼žĻĀ(T2)£¬·“Ó¦µÄĘ½ŗā³£ŹżK= ”££Ø½į¹ū±£ĮōŠ”ŹżµćŗóĮ½Ī»£©
£Ø2£©ŅŃÖŖ£ŗN2(g)£«O2(g) 2NO(g) ¦¤H£½£«180.5 kJ”¤mol-1
2NO(g) ¦¤H£½£«180.5 kJ”¤mol-1
2H2(g)£«O2(g) 2H2O(g) ¦¤H£½£483.6 kJ”¤mol-1
2H2O(g) ¦¤H£½£483.6 kJ”¤mol-1
½ńÓŠ17 g°±Ęų£¬¼ŁÉčĘ侓߻ÆŃõ»ÆĶźČ«·“Ó¦£¬Éś³ÉŅ»Ńõ»ÆµŖĘųĢåŗĶĖ®ÕōĘų£¬ŌņøĆ¹ż³ĢÖŠĖł·Å³öµÄČČĮæĪŖ kJ”£
£Ø3£©ŌŚ×°ÖĆ¢ŚÖŠ£¬NH3ŗĶO2“Ó145”ę¾ĶæŖŹ¼ĻĀĮŠ·“Ó¦£¬ŌŚ²»Ķ¬ĪĀ¶ČŗĶ“߻ƼĮĢõ¼žĻĀÉś³É²»Ķ¬²śĪļ£ØČēĻĀĶ¼ĖłŹ¾£©£ŗ
ĪĀ¶Č½ĻµĶŹ±Éś³É ĪŖÖ÷£¬ĪĀ¶ČøßÓŚ900”ꏱ£¬NO²śĀŹĻĀ½µµÄæÉÄÜŌŅņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com