
����Ŀ��ˮ�е��ܽ�����DO���Ķ����Ǻ���ˮ��ˮ�ʵ���Ҫָ�ꡣij��ѧС��ⶨij���������ĺ������������й������˽�ܽ����ⶨ����������������
������ȷ��������������ƣ�Na2S2O3����������һ�������cmol/L����Һ��
����ˮ��ƿȡ������ˮ��v1mL������������ע��1.0mLMnCl2��Һ��1.0mL����KI��Һ������ƿ����ƿ�ڲ������ݣ�����������Լ1Сʱ��
����ˮ��ƿ�м���1.0mL������Һ������ƿ������ˮ��ƿ������ȫ���ܽ⣬��ʱ��Һ��Ϊ��ɫ�� ������ˮ��ƿ����Һȫ��������ƿ�У�����������Ʊ���Һ�ζ���
V������Һ�ʵ���ɫ��1mL������Һ�������ζ����յ㲢��¼���ĵ������������Һ���Ϊv2��
��֪��I2 +2Na2S2O3 =2NaI+Na2S4O6
��1���ڵζ�������ʹ�õ������еζ��ܼС�����̨���ձ�����ƿ��________________________��
��2���ڲ�����У�ˮ���г�����MnMnO3���������ӷ���ʽΪ4Mn2++O2+8OH��![]() 2MnMnO3��+4H2O��
2MnMnO3��+4H2O��
��3��������з�����Ӧ�����ӷ���ʽΪ _______________________________________________________________��
��4���ζ�ʱ����Һ��__________ɫ��______________ɫ���Ұ��������ɫ���ٱ仯���ﵽ�ζ��յ㡣
��5����ˮ�е��ܽ���Ϊ_____________________________mg/L��
��6������ˮ�к��н϶�NO3��ʱ���ⶨ������ʵ��ֵ________(��ƫ�ߡ�ƫ�ͻ�)
���𰸡���ʽ�ζ��� MnMnO3+2I��+6H+![]() I2+2Mn2++3H2O �� ��
I2+2Mn2++3H2O �� �� ![]() ƫ��
ƫ��
��������
(1)�ζ�������ʹ�õ������еζ��ܼС�����̨���ձ�����ƿ�͵ζ��ܣ��ζ���װNa2S2O3��Һ��Na2S2O3�Լ��ԣ�
(3)������MnMnO3��������ͬʱ�е�����ʣ�࣬����1.0mL������Һ������ƿ������ˮ��ƿ������ȫ���ܽ⣬��ʱ��Һ��Ϊ��ɫ��˵������I2������������ԭ��Ӧ����ʽ������
(4)����Һ����I2���õ�����Һ��ָʾ������ҺΪ��ɫ���յ�ʱΪ��ɫ��
(5)���ݹ�ϵʽ�����ж���������
(6)���н϶�NO3��ʱ�������������£��γ����ᣬ����ǿ�����ԣ�������Na2S2O3��
(1)�ζ�������ʹ�õ������еζ��ܼС�����̨���ձ�����ƿ�͵ζ��ܣ��ζ���װNa2S2O3��Һ��Na2S2O3�Լ��ԣ��ü�ʽ�ζ��ܣ��ʴ�Ϊ����ʽ�ζ��ܣ�
(3)������MnMnO3��������ͬʱ�е�����ʣ�࣬����1.0mL������Һ������ƿ������ˮ��ƿ������ȫ���ܽ⣬��ʱ��Һ��Ϊ��ɫ��˵������I2�����⻯�ϼ����ߣ�Mn�Ļ��ϼۻή�ͣ����ӷ���ʽΪMnMnO3+2I��+6H+![]() I2+2Mn2++3H2O���ʴ�Ϊ��MnMnO3+2I��+6H+
I2+2Mn2++3H2O���ʴ�Ϊ��MnMnO3+2I��+6H+![]() I2+2Mn2++3H2O��
I2+2Mn2++3H2O��
(4)����Һ����I2���õ�����Һ��ָʾ������ҺΪ��ɫ���յ�ʱΪ��ɫ���ʴ�Ϊ�������ޣ�
(5)��4Mn2++O2+8OH��![]() 2MnMnO3��+4H2O��MnMnO3+2I��+6H+
2MnMnO3��+4H2O��MnMnO3+2I��+6H+![]() I2+2Mn2++3H2O��I2 +2Na2S2O3 =2NaI+Na2S4O6����֪��ϵʽ
I2+2Mn2++3H2O��I2 +2Na2S2O3 =2NaI+Na2S4O6����֪��ϵʽ![]() ����
���� �������������ʵ���x=
�������������ʵ���x=![]() ��v1mLˮ�����ܽ���=
��v1mLˮ�����ܽ���=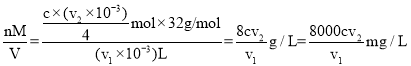 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(6)���н϶�NO3��ʱ�������������£��γ����ᣬ����ǿ�����ԣ�������Na2S2O3����Na2S2O3�������ӣ����ƫ�ߣ��ʴ�Ϊ��ƫ�ߡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ӷϾ���������ӵ�ص��������ϣ���������Ϳ����������LiCoO2���У������ܡ�﮵IJ�����������ͼ��ʾ��
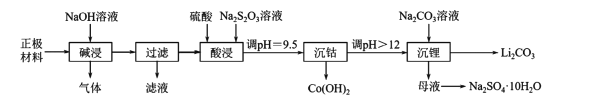
�ش��������⡣
��1�����Ͼɵ�ػ�ȡ��������ǰ���Ƚ������NaCl��Һ�У�ʹ��ض�·���ŵ磬��ʱ��Һ�¶����ߣ��ù�������������Ҫת����ʽΪ____��
��2��������������в�����������____�������ˡ�������Һ�����ᴦ���ɵõ�������������Ӧ�Ļ�ѧ����ʽΪ____��
��3���������ʱ��Ҫ��Ӧ�����ӷ���ʽΪ____�������ᡢNa2S2O3��Һ��һ��Ũ�ȵ����������Ҳ���Դﵽ���������Ŀ�ģ��������____���ѧʽ����Ⱦ������
��4�������ܡ�ʱ����pH���õ��Լ���____�������ܡ�����Һ��c��Co2+��=____������֪��Ksp[Co��OH��2]=1.09��l0-15��
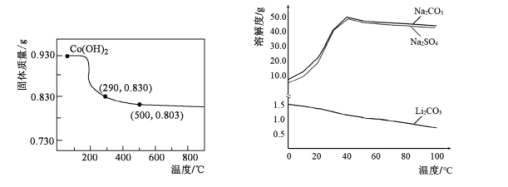
��5���ڿ����м���Co��OH��2��ʹ��ת��Ϊ�ܵ���������ȹ����У������������¶ȵĹ�ϵ������ͼ��ʾ��290��500�棬������Ӧ�Ļ�ѧ����ʽΪ____��
��6����������ͼ�жϣ�����ﮡ��л��Li2CO3����IJ�����Ҫ����____��____��ϴ�ӡ�����Ȳ��衣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ����������ȷ����(����)
A. ��ʾ��Ӧ4CO(g)+2NO2(g)N2(g)+4CO2(g)�����������������䣬�ı�CO�����ʵ�����ƽ��ʱN2����������仯�������ͼ��֪��NO2��ת���ʣ�c��b��a
��ʾ��Ӧ4CO(g)+2NO2(g)N2(g)+4CO2(g)�����������������䣬�ı�CO�����ʵ�����ƽ��ʱN2����������仯�������ͼ��֪��NO2��ת���ʣ�c��b��a
B. b��ʾ25��ʱ���ֱ��ˮϡ�������Ϊ100 mL��pH��2��CH3COOH��Һ��HX��Һ����25��ʱHX�ĵ���ƽ�ⳣ��С��CH3COOH�ĵ���ƽ�ⳣ��
b��ʾ25��ʱ���ֱ��ˮϡ�������Ϊ100 mL��pH��2��CH3COOH��Һ��HX��Һ����25��ʱHX�ĵ���ƽ�ⳣ��С��CH3COOH�ĵ���ƽ�ⳣ��
C. c��һ�������£�����һ����A������������Bʱ��ͼ��ѹǿp1 > p2
c��һ�������£�����һ����A������������Bʱ��ͼ��ѹǿp1 > p2
D. d����ƽ����ϵ����Һ����������KCl�����ѧ��Ӧ������ʱ��仯��ͼ��
d����ƽ����ϵ����Һ����������KCl�����ѧ��Ӧ������ʱ��仯��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��20��ʱ����0.1mol/L����ζ�20mL0.1mol/L��ˮ��ͼ����ͼ��ʾ������˵����ȷ����
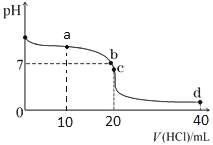
A.a��ʱ2c(Cl��)=c(NH3H2O)+c(NH4+)
B.b���ʾ���ǡ����ȫ��Ӧ
C.c��ʱc(NH4+)>c(Cl��)>c(H+)>c(OH��)
D.a��b��c��d����c(NH4+)+c(H+)=c(Cl��)+c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д��COS�ĵ���ʽ__________________��C��O�γɹ��ۼ�ʱ�����õ��ӶԻ�ƫ��__________ԭ�ӣ��ж�������___________��
��2����֪COS(g)��H2O(g)![]() H2S(g)��CO2(g) ��H1����34kJ/mol
H2S(g)��CO2(g) ��H1����34kJ/mol
CO(g)��H2O(g)![]() H2(g)��CO2(g) ��H2����41kJ/mol
H2(g)��CO2(g) ��H2����41kJ/mol
д��H2S��CO��Ӧ����COS���Ȼ�ѧ����ʽ__________________________________��
100��ʱ��CO��H2S�����ʵ�����Ϊ1��1���뷴Ӧ���У���ƽ���CO��ת������=33.3%����ʱ��Ӧ��ƽ�ⳣ��k=________________________��
��3���ڳ��д����ĺ�ѹ�ܱ������н��з�Ӧ������ʼ�����n(CO)��n(H2S)��m����ͬʱ���ڲ��H2Sת������m���¶ȣ�T���Ĺ�ϵ��ͼ��ʾ��
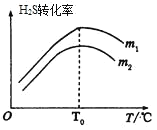
��m1________m2���������
���¶ȸ���T0ʱ��H2Sת���ʼ�С�Ŀ���ԭ��Ϊ_________
a����Ӧֹͣ�� b����Ӧ�ġ�H���
c����Ӧ�ﵽƽ�� d���������Խ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���е���ɫ�仯����������ԭ��Ӧ�ص���
ѡ�� | A | B | C | D |
ʵ�� | NaOH��Һ����FeSO4��Һ�� | ʯ����Һ������ˮ�� | KSCN��Һ����FeCl3��Һ�� | CO2ͨ��װ��Na2O2����ĸ���� |
���� | ������ɫ����������Ϊ���ɫ | ��Һ��죬���Ѹ����ɫ | ��Һ��Ϊ��ɫ | �����ɵ���ɫ��Ϊ��ɫ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҿ���NaBr��ŨH2SO4���Ҵ�Ϊԭ���Ʊ����������飺
C2H5��OH+HBr![]() C2H5Br+H2O
C2H5Br+H2O
��֪��Ӧ�������Ϊ��0.30 mol NaBr��s����0.25 mol C2H5OH���ܶ�Ϊ0.80 g��cm-3����36 mLŨH2SO4����������Ϊ98%���ܶ�Ϊ1.84 g��mL-1����25 mLˮ���Իش��������⡣
��1����ʵ��Ӧѡ��ͼ�е�aװ�û���bװ�ã�_____________��
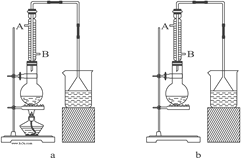
��2����Ӧװ���е���ƿӦѡ���������ֹ���������_____��
A.50 mL B.100 mL C.150 mL D.250 mL
��3���������е�����ˮ������Ӧ����_____��
A. A��B�� B. B��A�� C. ��A����B������
��4�����ܷ����ĸ���ӦΪ��_____________��__________��______________������д��3������ʽ����
��5��ʵ����ɺ��뽫��ƿ�ڵ��л�������������õ��ػ�ɫ�Ĵ������飬���ô��������飬Ӧ���õĴ�ʩ��_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ��ԭ���������������ǰ������Ԫ�ء�
��ԭ���������������ǰ������Ԫ�ء�![]() ����������ḻ��Ԫ�أ�
����������ḻ��Ԫ�أ�![]() Ԫ��ԭ�ӻ�̬ʱ��������Ӿ��Ų���3���ܼ��ϣ������ǵļ۵��Ӳ��Ͼ�������δ�ɶԵ��ӣ���
Ԫ��ԭ�ӻ�̬ʱ��������Ӿ��Ų���3���ܼ��ϣ������ǵļ۵��Ӳ��Ͼ�������δ�ɶԵ��ӣ���![]() ����Һ�еμӰ�ˮ���γ���ɫ�������ٵμӰ�ˮ�������ܽ⣬�õ�����ɫ��Һ���ش��������⣺
����Һ�еμӰ�ˮ���γ���ɫ�������ٵμӰ�ˮ�������ܽ⣬�õ�����ɫ��Һ���ش��������⣺
��1����һ������![]() ____
____![]() ���縺��
���縺��![]() ____
____![]() ��������������������������������
��������������������������������
��2��д����![]() ���ӻ�Ϊ�ȵ������һ�����ӵĻ�ѧʽ_________��
���ӻ�Ϊ�ȵ������һ�����ӵĻ�ѧʽ_________��
��3��������![]() ����������Һ�еμӹ�����ˮ���õ�����ɫ��Һ���ټ��Ҵ�����____________ɫ�����������þ����У��������ӵĵ����Ų�ʽΪ____________������Ϊ____________��
����������Һ�еμӹ�����ˮ���õ�����ɫ��Һ���ټ��Ҵ�����____________ɫ�����������þ����У��������ӵĵ����Ų�ʽΪ____________������Ϊ____________��
��4����֪![]() �γɵ�һ���Ԫ�����ﻯѧʽ��
�γɵ�һ���Ԫ�����ﻯѧʽ��![]() ��
��![]() ��
��![]() ��
��![]() ����
����![]() ������
������![]() ԭ�ӵ��ӻ�����Ϊ___________�����黯�����ͨʽΪ________________��
ԭ�ӵ��ӻ�����Ϊ___________�����黯�����ͨʽΪ________________��
��5����֪![]() ��
��![]() �����γ�
�����γ�![]() ��
��![]() ���ֻ�����Ը����й���Ϣ����������⣺
���ֻ�����Ը����й���Ϣ����������⣺
�������ģ�ͱ�ʾ��![]() ���ӽṹ��_______________��
���ӽṹ��_______________��
A.![]() B.
B.![]() C.
C.![]() D.
D.
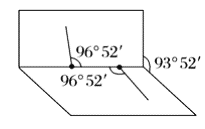
����֪![]() �ӵĽṹ��ͼ��ʾ��
�ӵĽṹ��ͼ��ʾ��![]() ���Ӳ���ֱ���εģ�����
���Ӳ���ֱ���εģ�����![]() ԭ�������ڰ�չ������������ϣ�����
ԭ�������ڰ�չ������������ϣ�����![]() ԭ�����鼹λ���ϣ���ҳ�н�Ϊ
ԭ�����鼹λ���ϣ���ҳ�н�Ϊ![]() ����
����![]() ����
����![]() ���ļнǾ�Ϊ
���ļнǾ�Ϊ![]() ����
����![]() �ӵĵ���ʽΪ_________���ṹʽΪ_________��
�ӵĵ���ʽΪ_________���ṹʽΪ_________��![]() ������_______�����������������Ǽ����������ӡ�
������_______�����������������Ǽ����������ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��WΪ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Yԭ�ӵ������������ڲ��������3��������˵����ȷ���� (����)
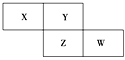
A. ԭ�Ӱ뾶��W>Z>XB. �ǽ����ԣ�Z>Y
C. ����ϼۣ�X>ZD. ����������Ӧˮ��������ԣ�W>Z
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com