

��ش��������⣺
(1)������ת����Ϊ��ԭ�����dz������������dz����ǽ������ʣ�������ʹ�����ǵ�ľ����ȼ���Ҽס��ҡ����ɶ�����Ԫ����ɡ���A�Ľṹʽ��__________________��
A���Ӧ�Ļ�ѧ����ʽ��____________________________________��
(2)������ת����Ϊ���������dz����ǽ�����̬���ʣ����dz����������ס��ҡ�����ֻ�м��ɶ�����Ԫ����ɣ��������ͬ���壬���ͬ���ڣ����dz�����Ψһ��Һ��ǽ������ʣ�Ҳ��ǿ�����ԡ�ͨ�������·�Ӧ������Һ�н��С���
�ٵ��ʱ���(дԪ�ط���)____________________________________��
��A���ҷ�Ӧ�����ӷ���ʽΪ____________________________________��
�۵�A������ʵ���֮��Ϊ2��3ʱ��A���ǡ����ȫ��Ӧ����������ͼ��ʾ����A����Һ����Ӧ�Ļ�ѧ����ʽΪ ____________________________________��
(1)O=C=O
2Mg+CO2 ![]() 2MgO+C
2MgO+C
(2)��Fe
��2Fe2++Br2![]() 2Fe3++2Br-
2Fe3++2Br-
��2FeBr2+3Cl2![]() 2FeCl3+2Br2
2FeCl3+2Br2
������(1)������ʹ�����ǵ�ľ����ȼ��Ϊ������������Ϊ̼������ȫȼ��ʱ���ɻ�����C��C��CO���壬��ȫȼ������CO2��������A��CO2����֪��������BΪþ����ӦΪ��2Mg+CO2![]() 2MgO+C��(2)���dz�����Ψһ��Һ��ǽ������ʣ�Ϊ�����壬���Ϊ���������dz���������Ϊ����������ʱ�����嵥�ʷ�Ӧ����FeBr3������ʱ����FeBr2����֪C��FeBr3��A��FeBr2��
2MgO+C��(2)���dz�����Ψһ��Һ��ǽ������ʣ�Ϊ�����壬���Ϊ���������dz���������Ϊ����������ʱ�����嵥�ʷ�Ӧ����FeBr3������ʱ����FeBr2����֪C��FeBr3��A��FeBr2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� |
| ���� |
| ���� |
| ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
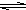 HS-+OH-
HS-+OH-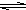 HS-+OH-
HS-+OH-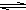 Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������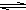 Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��
��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��
| ||
| �� |
| ||
| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� |
| �� |
| ���� |
| �� |
| ||
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com