
 N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1��
N2O4(g) ��H <0��ƽ�ⳣ�� K��13.3������������ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ���� c (NO2) =" 0.0300" mol��L��1�� CH3OH����Ӧ��CO(g)��2H2(g)
CH3OH����Ӧ��CO(g)��2H2(g) CH3OH(l) ��H =��131.9 kJ��mol��1���ͷ�Ӧ��2H2 (g) + CO(g) + 3/2O2g) =CO2 (g) +2H20 (g) ��H =��594.1 kJ��mol��1�����Ը��ݸ�˹���ɿ�֪�����ڣ��٣���2���õ� 2CH3OH(l) + 3O2(g) = 2CO2(g) + 4H2O(g)�����Է�Ӧ�ȡ�H =����594.1 kJ��mol��1��131.9 kJ��mol��1����2����1452 kJ��mol��1��
CH3OH(l) ��H =��131.9 kJ��mol��1���ͷ�Ӧ��2H2 (g) + CO(g) + 3/2O2g) =CO2 (g) +2H20 (g) ��H =��594.1 kJ��mol��1�����Ը��ݸ�˹���ɿ�֪�����ڣ��٣���2���õ� 2CH3OH(l) + 3O2(g) = 2CO2(g) + 4H2O(g)�����Է�Ӧ�ȡ�H =����594.1 kJ��mol��1��131.9 kJ��mol��1����2����1452 kJ��mol��1��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 CH(g)��H2(g) ��H1="156.6" kJ/mol
CH(g)��H2(g) ��H1="156.6" kJ/mol
 CH2(g)=CH4(g)��HC
CH2(g)=CH4(g)��HC
 CH(g ) ��H2="32.4" kJ/mol
CH(g ) ��H2="32.4" kJ/mol CH2(g)��H2(g)�ġ�H= kJ/mol��
CH2(g)��H2(g)�ġ�H= kJ/mol�� HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6��
HCO3����H����ƽ�ⳣ��K1= ������֪10-5.60=2.5��10-6���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
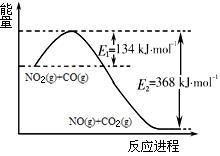
 2NH3(g) ��H < 0 ����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g) ��H < 0 ����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K | 4.1��106 | K1 | K2 |
 2NO2(g) ��H > 0 �����¶ȷֱ�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯��������ͼ��ʾ������˵����ȷ���� ��
2NO2(g) ��H > 0 �����¶ȷֱ�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯��������ͼ��ʾ������˵����ȷ���� ��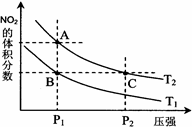
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
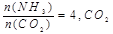 ��ת������ʱ��ı仯��ϵ��ͼ1��ʾ��
��ת������ʱ��ı仯��ϵ��ͼ1��ʾ�� ������ΪV����CO2��������ڡ�����С�ڡ����ڡ���
������ΪV����CO2��������ڡ�����С�ڡ����ڡ���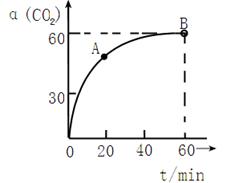
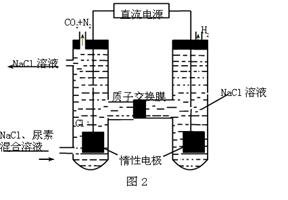
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ӦC(s)��CO2(g)��2CO(g)����H��0�����κ������¾������Է����� |
| B��Ǧ�����ڷŵ�����У���Һ��PHֵ���� |
| C�������£���0.1mol/L��ˮ�У���������NH4Cl���壬��Һ��pH��С |
| D���¶�һ��ʱ��ˮ�����ӻ�����Kw��������ᣨ�Ũ�ȵĸı���ı� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g) ��H1
CH3OH(g) ��H1 CH3OH(g) + H2O(g) ��H2
CH3OH(g) + H2O(g) ��H2| �¶� | 250�� | 300�� | 350�� |
| K | 2.041 | 0.270 | 0.012 |
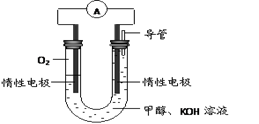
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A����ӦA��g�� 2B��g������H��������Ӧ�Ļ��ΪEa kJ mol-1���淴Ӧ�Ļ��ΪEb kJ��mol-1�����H=��Ea��Eb��kJ��mol-1 2B��g������H��������Ӧ�Ļ��ΪEa kJ mol-1���淴Ӧ�Ļ��ΪEb kJ��mol-1�����H=��Ea��Eb��kJ��mol-1 |
| B����֪25��ʱ���й�����ĵ���ƽ�ⳣ����HCN Ka=4.9��10-10�� H2CO3 Ka1=4.3��10-7��Ka2=5.6��10-11����CO2ͨ��NaCN��Һ�з�Ӧ�Ļ�ѧ����ʽΪ��2NaCN+H2O+CO2=2HCN+Na2CO3 |
C����֪�� ��Ӧ  ���ʱ�Ϊ��H = ��384 kJ��mol-1 ���ʱ�Ϊ��H = ��384 kJ��mol-1 |
| D��һ��Ũ�ȵ�NaOH��Һ���¶�����PHֵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڻ�ѧ��Ӧ�����У��������ʱ仯��ͬʱ��һ�����������仯 |
| B����101 kPaʱ��2 g H2��ȫȼ������Һ̬ˮ���ų�285.8 kJ���������ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ2H2(g)��O2(g)===2H2O(l)����H����571.6 kJ/mol |
| C���¶ȡ�Ũ�ȵĸı�һ��������Ӧ���ʵĸı䣬���Ի�ѧƽ��һ�����ƶ� |
| D�������ǡ����ȫ��Ӧ�������ε�c(H��)��c(OH��)��10��6 mol/L����Һһ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com