

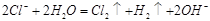



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
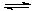 H++ SO42-
H++ SO42- µÄ·ĻĖ®»į·¢ÉśČēĻĀ·“Ó¦£ŗ
µÄ·ĻĖ®»į·¢ÉśČēĻĀ·“Ó¦£ŗ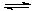 Mg(OH)2 Mg(OH)2+2NH4+
Mg(OH)2 Mg(OH)2+2NH4+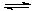 Mg2+ +2NH3”¤H2O”£
Mg2+ +2NH3”¤H2O”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 Ļņ
Ļņ µÄ×Ŗ»Æ£¬ĒėÄćĢį³öŅ»ĢõæÉŠŠŠŌ½ØŅé ”£
µÄ×Ŗ»Æ£¬ĒėÄćĢį³öŅ»ĢõæÉŠŠŠŌ½ØŅé ”£ 2CO2+N2”£
2CO2+N2”£ O2(g)=CO2(g) ”÷H=-283.0kJ”¤mol-1
O2(g)=CO2(g) ”÷H=-283.0kJ”¤mol-1| Ź±¼ä/s | 0 | 2 | 3 | 4 |
| c(NO)/mol”¤L-1 | 1.00”Į10-3 | 1.50”Į10-4 | 1.00”Į10-4 | 1.00”Į10-4 |
 C(CO)/mol”¤L-1 C(CO)/mol”¤L-1 | |  |  2.70”Į10-3 2.70”Į10-3 | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 O2(g)£½ZnO(s) ”÷H £½£351.1kJ”¤mol£1
O2(g)£½ZnO(s) ”÷H £½£351.1kJ”¤mol£1 O2(g)£½HgO(s) ”÷H £½£90.7 kJ”¤mol£1
O2(g)£½HgO(s) ”÷H £½£90.7 kJ”¤mol£1| A£®£260.4 kJ”¤mol£1 | B£®£441.8 kJ”¤mol£1 |
| C£®260.4 kJ”¤mol£1 | D£®441.8 kJ”¤mol£1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®CH3OH£Øl£©+ 3/2O2£Øg£©=CO2£Øg£©+ 2H2O£Øl£©£»”÷H="+725.8" kJ”¤mol£1 |
| B£®2CH3OH£Øl£©+ 3O2£Øg£©= 2CO2£Øg£©+ 4H2O£Øl£©£»”÷H="-1452" kJ”¤mol£1 |
| C£®2CH3OH£Øl£©+ 3O2£Øg£©= 2CO2£Øg£©+ 4H2O£Øl£©£»”÷H="-725.8" kJ”¤mol£1 |
| D£®2CH3OH£Øl£©+ 3O2£Øg£©= 2CO2£Øg£©+ 4H2O£Øl£©£»”÷H="+1452" kJ”¤mol£1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 2NH3µÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£¬
2NH3µÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£¬
 N2(g)+
N2(g)+ H2(g)
H2(g)  NH3(g ) ”÷H= £»
NH3(g ) ”÷H= £»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 2NO (g)£¬øĆ·“Ó¦ŹĒµ¼ÖĀĘū³µĪ²ĘųÖŠŗ¬ÓŠNOµÄŌŅņÖ®Ņ»”£
2NO (g)£¬øĆ·“Ó¦ŹĒµ¼ÖĀĘū³µĪ²ĘųÖŠŗ¬ÓŠNOµÄŌŅņÖ®Ņ»”£
 N2(g)£«O2(g)”£·“Ó¦¹ż³ĢÖŠN2µÄĢå»ż·ÖŹżĖꏱ¼ä±ä»ÆµÄĶ¼Ļń”£ÉżøßĪĀ¶Č£¬Ōņ·“Ó¦N2 (g)£«O2(g)
N2(g)£«O2(g)”£·“Ó¦¹ż³ĢÖŠN2µÄĢå»ż·ÖŹżĖꏱ¼ä±ä»ÆµÄĶ¼Ļń”£ÉżøßĪĀ¶Č£¬Ōņ·“Ó¦N2 (g)£«O2(g) 2NO (g)µÄĘ½ŗā³£ŹżK½« £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©”£
2NO (g)µÄĘ½ŗā³£ŹżK½« £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©”£ 2NO (g)µÄĘ½ŗā³£ŹżKµÄŹżÖµĪŖ ”£øĆĪĀ¶ČĻĀ£¬ČōæŖŹ¼Ź±ĻņÉĻŹöČŻĘ÷ÖŠ³äČėN2ÓėO2¾łĪŖ1 mol£¬Ōņ“ļµ½Ę½ŗāŗóN2µÄ×Ŗ»ÆĀŹĪŖ ”£
2NO (g)µÄĘ½ŗā³£ŹżKµÄŹżÖµĪŖ ”£øĆĪĀ¶ČĻĀ£¬ČōæŖŹ¼Ź±ĻņÉĻŹöČŻĘ÷ÖŠ³äČėN2ÓėO2¾łĪŖ1 mol£¬Ōņ“ļµ½Ę½ŗāŗóN2µÄ×Ŗ»ÆĀŹĪŖ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®2H+(aq) +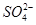 (aq)+ (aq)+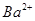 (aq)+2 (aq)+2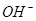 (aq) = BaSO4(s)+2H (aq) = BaSO4(s)+2H O(l); O(l);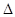 H = H =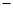 57.3 kJ/mol 57.3 kJ/mol |
B£®KOH(aq)+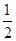 H H SO4(aq) = SO4(aq) = 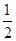 K K SO4(aq)+H SO4(aq)+H O(l); O(l);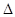 H= H=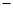 57.3kJ/mol 57.3kJ/mol |
C£®C8H18(l)+ 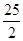 O O (g)=8CO (g)=8CO (g)+ 9H (g)+ 9H O(g); O(g);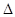 H= H=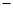 5518 kJ/mol 5518 kJ/mol |
D£®2C8H18(g)+25O (g)=16CO (g)=16CO (g)+18H (g)+18H O(1); O(1); H= H= 5518 kJ/mol 5518 kJ/mol |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com