
(ÿС��2�֣���3С��ȫ�ԲŸ��֣���10��)W��X��Y��Z��ԭ���������������ͬһ��ͬ��Ԫ�أ�W��X�ǽ���Ԫ�أ�Y��Z�Ƿǽ���Ԫ�ء�
(1��W��X���Ե�����������Ӧ��ˮ����������Ӧ���κ�ˮ���÷�Ӧ�����ӷ���ʽΪ____________________��
(2��W��Y ���γɻ�����W2Y���û�����ĵ���ʽΪ______________��
(3��X��������ˮ��Һ��______�ԣ������ӷ���ʽ����ԭ��________________________��
(4��Y�ĵͼ�������ͨ��Z���ʵ�ˮ��Һ�У�������Ӧ�Ļ�ѧ����ʽΪ_________________��
(5��Z�����������Ϊ��ɫҺ�壬0.25 mol��������һ����ˮ��ϵõ�һ��ϡ��Һ�����ų�QkJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ķ��ӽṹ�У���ÿ����2����ԭ�ӣ����൱��̼̼������һ�Թ��õ��Ӷԡ�
�Իش�
��1������ʽΪCnH2n+2����������̼̼�乲�е��Ӷ���Ϊ _��
��2������ʽΪCnH2n��6??����������̼̼�乲�õ��Ӷ���Ϊ ___ __��
��3��Cx�ɿ������������IJ����ij���ʷ�����̼̼��Ĺ��õ��Ӷ���Ϊ160������ϸ�������̼���ʵķ���ʽΪ �����ϸ������ĵ�ϩ���ķ���ʽΪ ��
��4��Ŀǰ����ѧ�����Ѿ��ҵ�ʮ���ָ���ϩ�����Cx�����Ƿ��ӽṹ��
������������κ��������ι��ɵķ�յ������壬C60�������е�һ�ָ���ϩ����ṹ��ͼ��ʾ��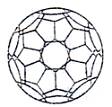 ��(3)С���е�CxҲ������һ�ָ���ϩ�����(3)С��Cx�ṹ������κ������εĸ����ֱ���____________�� ������ʾ��ŷ��������������+����������=2��
��(3)С���е�CxҲ������һ�ָ���ϩ�����(3)С��Cx�ṹ������κ������εĸ����ֱ���____________�� ������ʾ��ŷ��������������+����������=2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ��ɶ���УЭ�����һ��ѧ������������ѧ�Ծ����������� ���ͣ������
���ڣ�1��С��ÿ��1�֣�����ÿ��2�֣���12�֣�
A��B��C��D���ֶ�����Ԫ�أ�ԭ��������������A��Cͬ���壬C��Dͬ���ڣ�Bԭ�����������������ڲ��������3����A��B���γ�A2B��A2B2����Һ̬�����B��CҲ���γ�C2B��C2B2���ֹ�̬�����C����������ԭ����ͬ��������Ų���Dԭ��������������Bԭ��������������һ�����ӣ����������������жϣ�
��1������Ԫ�طֱ��ǣ�дԪ�ط��ţ� A B C D
��2���õ���ʽд��A2B��������γɹ���___�� �õ���ʽд��C2B��������γɹ���___��
��3��A2B2�м�����MnO2��Ӧ�Ļ�ѧ����ʽ________;C2B2��CO2��Ӧ�Ļ�ѧ����_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
����4С�⣬(ÿ��1�֣���4С��2�֣���10��)
��1��1mol H2SO4��Լ���� ��H2SO4�� mol H , mol O
��2��9.03��1023��CO2�����ʵ����� mol���ڱ�״���µ���� L���������� �ˡ�
��3����100mL2mol/LH2SO4��Һ�У�H2SO4�������� �ˡ�H+�����ʵ���Ũ��Ϊ ��
��4����������Ϊ36.5�����ܶ�Ϊ1.18g/cm3��Ũ������HCl�����ʵ���Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��ÿ��1�֣���4�֣���ͨ��״���£���ͬѧȡ1 mol H2O���ȵ�100��ʱ��Һ̬ˮ������Ϊˮ��������ͼ��ʾ�����ù������� �仯��
�ڱ���ѹǿ���������£�ˮ��������� 
���������������������22.4L��
����ͬѧ��H2��O2��ȼ�յ�ʵ�飬��ʵ��������� �仯���ڸñ仯�����У�һ��������ȵ��� ������ţ���
| A����Ӧ�������Ŀ�������������Ŀ | B����Ӧ��ԭ�������ʵ�����������ԭ�������ʵ��� |
| C����Ӧ���������������������� | D����Ӧ���������������� |

 �ۿ̶��� ��Ũ�� ���ݻ�
�ۿ̶��� ��Ũ�� ���ݻ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com