
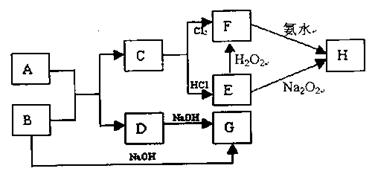



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ԭ�������ɴ�С��˳��ΪZ��Y��X |
| B��YԪ������������Ӧˮ����Ļ�ѧʽ�ɱ�ʾΪH3YO4 |
| C��3��Ԫ�ص���̬�⻯����Z����̬�⻯�����ȶ� |
| D��ԭ�Ӱ뾶�ɴ�С��˳��ΪZ��Y��X |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Ԫ�ؾ����ڵ������ڣ��Ҿ�������Ԫ�� |
| B��H2Se��H2O���ȶ��Ժ� |
| C�����ʸ��ܴ�FeSO4��Һ���û����� |
| D������ƷΪ�����ṩ�˱����Fe��Zn��Se����Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��������֮����E����������ȣ�B��C��������Ϊ������D��Eλ��ͬһ���ڣ�D������������������������ڸ������ӵĵ��Ӳ������Իش��������⣺
��������֮����E����������ȣ�B��C��������Ϊ������D��Eλ��ͬһ���ڣ�D������������������������ڸ������ӵĵ��Ӳ������Իش��������⣺ һ�š��������õ�Һ��ȼ�������� ����Ԫ�����ƣ�Ԫ�صĵ��ʣ�
һ�š��������õ�Һ��ȼ�������� ����Ԫ�����ƣ�Ԫ�صĵ��ʣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
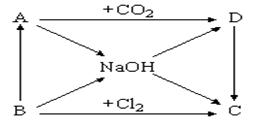
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��
�� ��B��ϡ��Һ��AgNO3��Һ��Ͽ��γɳ���AgX���˳�����Ksp(AgX) =1.8��10��10�����������Bϡ��Һ��AgNO3��Һ��ϣ���B��Ũ��Ϊ2��10��4
��B��ϡ��Һ��AgNO3��Һ��Ͽ��γɳ���AgX���˳�����Ksp(AgX) =1.8��10��10�����������Bϡ��Һ��AgNO3��Һ��ϣ���B��Ũ��Ϊ2��10��4 mo1/L
mo1/L  �������ɳ�������AgNO3��Һ����СŨ��Ϊ________������AgX����Һ�еμ�KI��Һ���۲쵽������ �������ܹ�����ת����ԭ���� ��
�������ɳ�������AgNO3��Һ����СŨ��Ϊ________������AgX����Һ�еμ�KI��Һ���۲쵽������ �������ܹ�����ת����ԭ���� ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com