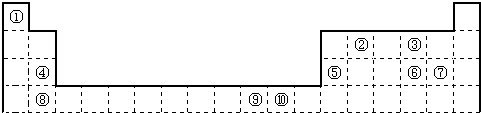
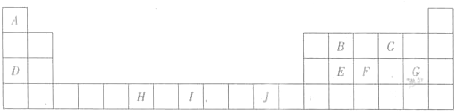
£Ø2012?ŃÓ±ßÖŻÄ£Äā£©ČēĶ¼1ĪŖ³¤Ź½ÖÜĘŚ±ķµÄŅ»²æ·Ö£¬ĘäÖŠµÄ±ąŗÅ“ś±ķ¶ŌÓ¦ŌŖĖŲ£®

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŖĖŲ¢ŚµÄŅ»ÖÖĒā»ÆĪļŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬³£°ŃøĆĒā»ÆĪļµÄ²śĮæ×÷ĪŖŗāĮæŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½µÄ±źÖ¾£®ÓŠ¹ŲøĆĒā»ÆĪļ·Ö×ÓµÄĖµ·ØÕżČ·µÄŹĒ
BD
BD
£®
A£®·Ö×ÓÖŠŗ¬ÓŠĒā¼ü
B£®ŹōÓŚŗ¬ÓŠ¼«ŠŌ¼üµÄ·Ē¼«ŠŌ·Ö×Ó
C£®Ö»ŗ¬ÓŠ4øö¦Ņ¼üŗĶ1øö¦Š¼ü
D£®øĆĒā»ÆĪļ·Ö×ÓÖŠŌŖĖŲ¢ŚŌ×Ó²ÉÓĆsp
2ŌÓ»Æ
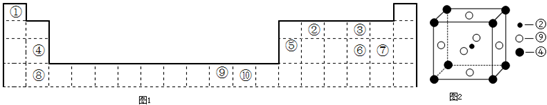
£Ø2£©æĘѧ·¢ĻÖ£¬¢Ś”¢¢Ż”¢¢įČżÖÖŌŖĖŲµÄŌ×ÓŠĪ³ÉµÄ¾§Ģå¾ßÓŠ³¬µ¼ŠŌ£¬Ę侧°ūµÄ½į¹¹ČēĶ¼2ĖłŹ¾£ØĶ¼ÖŠ¢Ś”¢¢Ż”¢¢į·Ö±šĪ»ÓŚ¾§°ūµÄĢåŠÄ”¢¶„µć”¢ĆęŠÄ£©£¬ŌņøĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ
MgNi3C
MgNi3C
£ØÓƶŌÓ¦µÄŌŖĖŲ·ūŗűķŹ¾£©£®
£Ø3£©a£ŗ±Č½Ļ¢Ś”¢¢Ū”¢¢ÜČżÖÖŌŖĖŲµŚŅ»µēĄėÄܓ󊔣¬ÓÉŠ”µ½“óÅÅĮŠµÄĖ³ŠņĪŖ
C£¼O£¼N
C£¼O£¼N
£ØÓĆŌŖĖŲ·ūŗűķŹ¾£©£®
b£ŗ±Č½Ļ¢Ü”¢¢Ž”¢¢ß”¢¢ąĖÄÖÖŌŖĖŲµÄµēøŗŠŌ“󊔣¬Óɓ󵽊”ÅÅĮŠµÄĖ³ŠņĪŖ
O£¾Cl£¾S£¾Al
O£¾Cl£¾S£¾Al
£ØÓĆŌŖĖŲ·ūŗűķŹ¾£©£®
£Ø4£©Ä³ŌŖĖŲµÄ¼Ūµē×ÓÅŲ¼Ź½ĪŖns
nnp
n+1£¬øĆŌŖĖŲÓėŌŖĖŲ¢ŁŠĪ³ÉµÄ18µē×ÓµÄX·Ö×ӵĽį¹¹Ź½ĪŖ
£»øĆŌŖĖŲ»¹æÉÓėŌŖĖŲ¢ŁŠĪ³É10µē×ÓµÄĘųĢå·Ö×ÓY£¬½«¹żĮæµÄYĘųĢåĶØČėŹ¢ÓŠŗ¬¢āŌŖĖŲµÄĮņĖįŃĪČÜŅŗÖŠ£¬·“Ó¦¹ż³ĢÖŠµÄŹµŃéĻÖĻóĪŖ£ŗ
ĻČÉś³ÉĄ¶É«³Įµķ£¬ŗó³ĮµķČܽā£¬ČÜŅŗ³ŹÉīĄ¶É«
ĻČÉś³ÉĄ¶É«³Įµķ£¬ŗó³ĮµķČܽā£¬ČÜŅŗ³ŹÉīĄ¶É«
£»·“Ó¦¹ż³ĢÖŠµÄĄė×Ó·½³ĢŹ½ĪŖ
Cu2++2 NH3?H2O=Cu£ØOH£©2”ż+2 NH4+”¢Cu£ØOH£©2+4NH3=[Cu£ØNH3£©4]2++2OH-
Cu2++2 NH3?H2O=Cu£ØOH£©2”ż+2 NH4+”¢Cu£ØOH£©2+4NH3=[Cu£ØNH3£©4]2++2OH-
£®
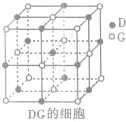
£Ø5£©¢āŌŖĖŲµ„ÖŹ¾§ĢåŹĒĆęŠÄĮ¢·½Ģ壬Į¢·½ĢåµÄĆæøöĆę5øö¢āŌ×Ó½ōĆܶŃĘö£¬¢āŌ×Ó°ė¾¶ĪŖd cm£¬øĆ½šŹōµÄĆܶČĪŖ
g?cm
-3£®£ØøĆ½šŹōŌ×ÓµÄÖŹĮæĪŖa g£©

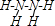 £»
£»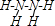 £»ĻČ²śÉśĄ¶É«³Įµķ£¬ŗó³ĮĻūŹ§£¬±ä³ÉÉīĄ¶É«µÄČÜŅŗ£»Cu2++2NH3?H2O=Cu£ØOH£©2”ż+2NH4+”¢Cu£ØOH£©2+4NH3=[Cu£ØNH3£©4]2++2OH-£»
£»ĻČ²śÉśĄ¶É«³Įµķ£¬ŗó³ĮĻūŹ§£¬±ä³ÉÉīĄ¶É«µÄČÜŅŗ£»Cu2++2NH3?H2O=Cu£ØOH£©2”ż+2NH4+”¢Cu£ØOH£©2+4NH3=[Cu£ØNH3£©4]2++2OH-£»

 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø



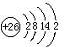
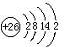
 £Ø3£©H3+Ąė×ÓŌŚŅ»¶ØĢõ¼žĻĀÄÜŠĪ³É½į¹¹ø“ŌÓµÄÅäĄė×Ó[HG£ØA2C£©5]2+ŠĪ³ÉøĆÅäĄė×ÓŹ±£¬H3+Ąė×Ó½ÓŹÜĮĖÅäĢåĢį¹©µÄ
£Ø3£©H3+Ąė×ÓŌŚŅ»¶ØĢõ¼žĻĀÄÜŠĪ³É½į¹¹ø“ŌÓµÄÅäĄė×Ó[HG£ØA2C£©5]2+ŠĪ³ÉøĆÅäĄė×ÓŹ±£¬H3+Ąė×Ó½ÓŹÜĮĖÅäĢåĢį¹©µÄ