
ĪŽ»ś»ÆŗĻĪļAÖŠŗ¬ÓŠŌŖĖŲLiŌŖĖŲ£¬AµÄĦ¶ūÖŹĮæ23g·mol-1£¬AÖ÷ŅŖÓĆÓŚÓŠ»śŗĻ³ÉŗĶŅ©ĪļÖĘŌģ£¬Ķ¬Ź±Ņ²ŹĒĮ¼ŗƵē¢Ēā²ÄĮĻ”£ŌŚŅ»¶ØĢõ¼žĻĀ£¬0.1mol¹ĢĢåAÓė0.1molNH4Cl¹ĢĢåĒ”ŗĆĶźČ«·“Ó¦£¬Éś³É¹ĢĢåBŗĶ4.48L(±ź×¼×“æö)ĘųĢåC”£ŅŃÖŖĘųĢåC¼«Ņ×ČÜÓŚĖ®£¬ĒŅµĆµ½¼īŠŌČÜŅŗ”£µē½āĪŽĖ®BæÉÉś³É½šŹōµ„ÖŹDŗĶĀČĘų”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄ»Æѧ·½Ź½ŹĒ_________________________”£
£Ø2£©Š“³ö»ÆŗĻĪļAÓėNH4Cl·“Ó¦µÄ»Æѧ·½³ĢŹ½:_______________________________”£
£Ø3£©Ä³Ķ¬Ń§Ķعż²éŌÄ׏ĮĻµĆÖŖĪļÖŹAµÄŠŌÖŹ£ŗ
I.¹¤ŅµÉĻæÉÓĆ½šŹōDÓėŅŗĢ¬µÄCŌŚĻõĖįĢś“ß»ÆĻĀ·“Ó¦Ą“ÖʱøAĪļÖŹ”£
II.ĪļÖŹAÓöĖ®ĒæĮŅĖ®½ā£¬ŹĶ·Å³öĘųĢåC”£
¢ŁIÖŠ·¢Éś·“Ó¦µÄ»ł±¾·“Ó¦ĄąŠĶŹĒ__________________________”£
¢ŚĪļÖŹAÓöĖ®ĒæĮŅĖ®½āµÄ»Æѧ·½³ĢŹ½ĪŖ_________________________”£
£Ø4£©¹¤ŅµÖʱøµ„ÖŹDµÄĮ÷³ĢČēĻĀ£ŗ
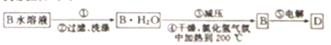
¢Ł²½Öč¢ŁÖŠ²Ł×÷µÄĆū³ĘĪŖ________________”£
¢ŚŹŌÓĆĘ½ŗāŌĄķ½āŹĶ²½Öč¢ŪÖŠ¼õŃ¹µÄÄæµÄŹĒ£ŗ_________________________”£
”¾ÖŖŹ¶µć”æĪŽ»śĪļµÄĶʶĻ£»ÖʱøŹµŃé·½°øµÄÉč¼Ę C1 J5
”¾“š°ø½āĪö”æ(1)LiNH2(2·Ö) £Ø2£©LiNH2£«NH4Cl=LiCl£«2NH3”ü
(3)¢ŁÖĆ»»·“Ó¦(1·Ö) ¢ŚLiNH2£«2H2O=LiOH£«NH3”ü(2·Ö)
(4)¢ŁÕō·¢ÅØĖõ”¢ĄäČ“½į¾§ LiCl·H2O(s) LiCl(s)£«H2O(g)£¬¼õŠ”Ń¹Ē棬ӊĄūÓŚĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬“Ó¶ųÓŠĄūÓŚĪŽĖ®LiClµÄÖʱø£Øø÷2·Ö£©
LiCl(s)£«H2O(g)£¬¼õŠ”Ń¹Ē棬ӊĄūÓŚĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬“Ó¶ųÓŠĄūÓŚĪŽĖ®LiClµÄÖʱø£Øø÷2·Ö£©
½āĪö£ŗŌŚŅ»¶ØĢõ¼žĻĀ£¬0.1mol¹ĢĢåAÓė0.1molNH4Cl¹ĢĢåĒ”ŗĆĶźČ«·“Ó¦£¬Éś³É¹ĢĢåBŗĶ4.48L(±ź×¼×“æö)ĘųĢåC£¬ĘųĢåC¼«Ņ×ČÜÓŚĖ®µĆµ½¼īŠŌČÜŅŗ£¬æÉĶĘÖŖCĪŖNH3£¬µē½āĪŽĖ®BæÉÉś³ÉŅ»ÖÖ¶ĢÖÜĘŚŌŖĖŲµÄ½šŹōµ„ÖŹDŗĶĀČĘų£¬BĪŖ½šŹōDµÄĀČ»ÆĪļ£¬4.48L°±ĘųµÄĪļÖŹµÄĮæ=4.48L/22.4L/mol=0.2mol£¬ĘäÖŹĮæ=0.2mol”Į17g/mol=3.4g£¬0.1mol¹ĢĢåAµÄÖŹĮæĪŖ2.30g£¬0.1molNH4Cl¹ĢĢåµÄÖŹĮæĪŖ5.35g£¬øł¾ŻÖŹĮæŹŲŗćæÉÖŖBµÄÖŹĮæĪŖ2.3g+5.35g-3.4g=4.25g£¬ AÖŠŗ¬Li£¬ŌņDĪŖ¢ńA×彚Źō£¬Ōņ¹ĢĢåAÓėNH4Cl¹ĢĢå·“Ó¦æɱķĪŖ£ŗA+NH4Cl”śLiCl+NH3£¬øł¾ŻClŌ×ÓŹŲŗć£¬LiClµÄĪļÖŹµÄĮæ=0.1mol£¬ÄĒĆ“2.3g»ÆŗĻĪļAÖŠŗ¬LiŌŖĖŲŅ²ĪŖ 0.1mol£¬ŌŁøł¾ŻÖŹĮæŹŲŗćŗĶŌ×ÓŹŲŗć£ØŌ×ÓµÄÖÖĄąŗĶŹżÄæ·“Ó¦Ē°ŗóĻąĶ¬£©£¬Ōņ2.3gAÖŠŗ¬ÓŠNŌ×ÓĪŖ0.2mol-0.1mol=0.1mol£¬ŗ¬ÓŠHŌ×ÓĪŖ0.2mol”Į4-0.4mol=0.2mol£¬æÉĶĘÖŖAŹĒLiNH2”£
£Ø1£©ÓÉÉĻŹö·ÖĪöæÉÖŖ£¬AĪŖLiNH2£¬CĪŖ°±Ęų£¬Ęäµē×ÓŹ½ĪŖ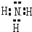 ”£
ӣ
£Ø2£©»ÆŗĻĪļAÓėNH4Cl·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖLiNH2£«NH4Cl=LiCl£«2NH3”ü
£Ø3£©¢Ł½šŹōLiÓėŅŗĢ¬µÄN3HŌŚĻõĖįĢś“ß»ÆĻĀ·“Ó¦Ą“ÖʱøLiNH2ĪļÖŹĶ¬Ź±Éś³ÉĮĖĒāĘų£¬¹Ź·“Ó¦ĪŖÖĆ»»·“Ó¦”£¢ŚĪļÖŹLiNH2ÓöĖ®·¢ÉśĖ®½ā£¬Ó¦ŹĒļ®Ąė×Ó½įŗĻĖ®µēĄė²śÉśµÄĒāŃõøłĄė×Ó£¬NH2—½įŗĻĖ®µēĄė²śÉśµÄĒāĄė×Ó£¬¹ŹĖ®½ā·“Ó¦·½³ĢŹ½ĪŖ£ŗLiNH2£«2H2O=LiOH£«NH3”ü”£
£Ø4£©¢ŁÓÉĮ÷³ĢæÉÖŖÓ¦ŹĒ“ÓČÜŅŗÖŠµĆµ½¾§Ģ壬Ōņ²½Öč¢ŁÖŠ²Ł×÷Ćū³ĘĪŖÕō·¢ÅØĖõ”¢ĄäČ“½į¾§£»¢ŚÓÉLiCl﹒H2O⇌LiCl+H2OæÉÖŖ£¬²½Öč¢ŚÖŠ¼õŃ¹µÄÄæµÄŹĒ¼õŠ”Ń¹Ē棬ӊĄūÓŚÉĻŹöĘ½ŗāĻņÕż·½ĻņŅĘ¶Æ£¬ÓŠĄūÓŚĪŽĖ®LiClµÄÖʱø”£
”¾Ė¼Ā·µć²¦”æ±¾Ģāæ¼²éĪŽ»śĪļĶʶĻ”¢»ÆѧŹµŃéµČ£¬ĢāÄæĖŲ²Ä±Č½ĻÄ°Éś£¬Ōö“óĢāÄæÄŃ¶Č£¬²ąÖŲæ¼²éѧɜ¶ŌÖŖŹ¶µÄĒØŅĘÓ¦ÓĆÓė×ŪŗĻ·ÖĪö½ā¾öĪŹĢāÄÜĮ¦£¬¶ŌѧɜµÄĀß¼ĶĘĄķÓŠ½ĻøßµÄŅŖĒ󣬰ŃĪÕAÖŠŗ¬LiŌŖĖŲ£¬Ōņ½šŹōDĪŖLiŹĒ¹Ų¼ü£¬ÄŃ¶Č½Ļ“ó”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤³§ÖŠÓĆĻ”ĮņĖį½žÅŻÄ³æóŹÆŗóµÄČÜŅŗÖŠ£¬³żĮĖŗ¬ÓŠ“óĮæĮņĖįĶā£¬»¹ŗ¬ÓŠÉŁĮæNH4+”¢Fe3£«”¢AsO43£”¢Cl£”£ĪŖ³żČ„ŌÓÖŹĄė×Ó£¬²æ·Ö²Ł×÷Į÷³ĢČēĻĀ£ŗ
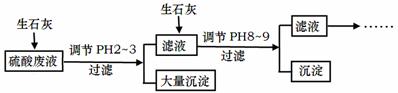
Ēė»Ų“šĪŹĢā£ŗ
£Ø1£©ÓĆĻ”ĮņĖį½žÅŻÄ³æóŹÆŗóµÄČÜŅŗÖŠ£¬ĮņĖįµÄÅضČĪŖ4.9g”¤L£1£¬ŌņøĆČÜŅŗÖŠµÄpHŌ¼ĪŖ ”£
£Ø2£©NH4+ŌŚÓĆĻ”ĮņĖį½žÅŻÄ³æóŹÆŗóµÄČÜŅŗÖŠŅŌ(NH4)2SO4ŗĶNH4ClŠĪŹ½“ęŌŚ”£ĻÖÓŠŅ»·Ż(NH4)2SO4ČÜŅŗ£¬Ņ»·ŻNH4ClČÜŅŗ£¬(NH4)2SO4ČÜŅŗÖŠc(NH4£«)Ē”ŗĆŹĒNH4ClČÜŅŗÖŠc(NH4£«)µÄ2±¶£¬Ōņc[(NH4)2SO4] c(NH4Cl)£ØĢī£ŗ£¼”¢=»ņ£¾£©”£
£Ø3£©Ėę×ÅĻņ·ĻŅŗÖŠĶ¶ČėÉśŹÆ»Ņ£ØŗöĀŌČÜŅŗĪĀ¶ČµÄ±ä»Æ£©£¬ČÜŅŗÖŠ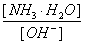 _______(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
_______(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
£Ø4£©Ķ¶ČėÉśŹÆ»Ņµ÷½ŚpHµ½2~3Ź±£¬“óĮæ³ĮµķÖ÷ŅŖ³É·ÖĪŖCaSO4”¤2H2O[ŗ¬ÓŠÉŁĮæFe(OH)3]£¬Ģį“æCaSO4”¤2H2OµÄÖ÷ŅŖ²Ł×÷²½Öč£ŗĻņ³ĮµķÖŠ¼ÓČė¹żĮæ £¬³ä·Ö·“Ó¦ŗ󣬹żĀĖ”¢Ļ“µÓ”¢ ”£
£Ø5£©25”ę, H3AsO4µēĄė³£ŹżĪŖK1=5.6”Į10£3£¬K2=1.7”Į10£7£¬K3=4.0”Į10£12”£µ±ČÜŅŗÖŠpHµ÷½Śµ½8~9Ź±£¬³ĮµķÖ÷ŅŖ³É·ÖĪŖCa3(AsO4)2”£
¢ŁpHµ÷½Śµ½8×óÓŅCa3(AsO4)2²ÅæŖŹ¼³ĮµķµÄŌŅņŹĒ ”£
¢ŚNa3AsO4µŚŅ»²½Ė®½āµÄĘ½ŗā³£ŹżŹżÖµĪŖ£ŗ ”£
¢ŪŅŃÖŖ£ŗAsO43£+2I£+2H+= AsO33£+I2+H2O £¬SO2+I2+2H2O=SO42£+2I£+4H+ ”£ÉĻŹöĮ½øö·“Ó¦ÖŠ»¹ŌŠŌ×īĒæµÄĪ¢Į£ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĪļÖŹ·ÖĄąÕżČ·µÄŹĒ
¢Ł»ģŗĻĪļ£ŗĀĮČČ¼Į”¢ø£¶ūĀķĮÖ”¢Ė®²£Į§”¢ĘÆ°×·Ū””¢Ś»ÆŗĻĪļ£ŗĀČ»ÆøĘ”¢ÉÕ¼ī”¢±łĖ®»ģŗĻĪļ”¢µØ·Æ””¢Ūµē½āÖŹ£ŗĆ÷·Æ”¢¶žŃõ»ÆĢ¼”¢±ł“×Ėį”¢ĮņĖį±µ””¢ÜĶ¬ĻµĪļ£ŗCH2O2”¢C2H4O2”¢C3H6O2”¢C4H8O2””¢ŻĶ¬ĖŲŅģŠĪĢå£ŗC60”¢C70”¢½šøÕŹÆ”¢ŹÆÄ«
A”¢¢Ł¢Ś¢Ü B”¢¢Ś¢Ū¢Ü C”¢¢Ł¢Ś¢Ż D”¢¢Ś¢Ü¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹żŃõ»ÆøĘæÉŅŌÓĆÓŚøÄÉʵŲ±ķĖ®ÖŹ£¬“¦Ąķŗ¬ÖŲ½šŹōĮ£×Ó·ĻĖ®ŗĶÖĪĄķ³ą³±£¬Ņ²æÉÓĆÓŚÓ¦¼±¹©ŃõµČ”£¹¤ŅµÉĻÉś²ś¹żŃõ»ÆøʵÄÖ÷ŅŖĮ÷³ĢČēĻĀ£ŗ

ŅŃÖŖCaO2·8H2O³Ź°×É«£¬Ī¢ČÜÓŚĖ®£¬¼ÓČČÖĮ350 ”ę×óÓŅæŖŹ¼·Ö½ā·Å³öŃõĘų”£
(1)ÓĆÉĻŹö·½·ØÖĘČ”CaO2·8H2OµÄ»Æѧ·½³ĢŹ½ŹĒ______________________________£»
(2)¼ģŃé”°Ė®Ļ“”±ŹĒ·ńŗĻøńµÄ·½·ØŹĒ___________________________________________£»
(3)³ĮµķŹ±³£ÓƱłĖ®æŲÖĘĪĀ¶ČŌŚ0 ”ę×óÓŅ£¬ĘäæÉÄÜŌŅņŹĒ______________________£»
(4)²ā¶Ø²śĘ·ÖŠCaO2µÄŗ¬ĮæµÄŹµŃé²½Öč£ŗ
µŚŅ»²½£ŗ×¼Č·³ĘČ”a g²śĘ·ÓŚÓŠČū׶ŠĪĘæÖŠ£¬¼ÓČėŹŹĮæÕōĮóĖ®ŗĶ¹żĮæµÄb g KI¾§Ģ壬ŌŁµĪČėÉŁĮæ2 mol·L£1µÄH2SO4ČÜŅŗ£¬³ä·Ö·“Ó¦”£
µŚ¶ž²½£ŗĻņÉĻŹö׶ŠĪĘæÖŠ¼ÓČė¼øµĪµķ·ŪČÜŅŗ”£
µŚČż²½£ŗÖšµĪ¼ÓČėÅضČĪŖc mol·L£1µÄNa2S2O3ČÜŅŗÖĮ·“Ó¦ĶźČ«£¬ĻūŗÄNa2S2O3ČÜŅŗV mL”£
”¾ŅŃÖŖ£ŗI2£«2S2O32-£½2I££«S4O62-”æ
¢ŁµŚŅ»²½·¢ÉśµÄ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»
CaO2µÄÖŹĮæ·ÖŹżĪŖ____________(ÓĆ×ÖÄø±ķŹ¾)£»
¢ŚÄ³Ķ¬Ń§µŚŅ»²½ŗĶµŚ¶ž²½µÄ²Ł×÷¶¼ŗÜ¹ę·¶£¬µŚČż²½µĪĖŁĢ«Āż£¬ÕāŃł²āµĆµÄCaO2µÄÖŹĮæ·ÖŹżæÉÄÜ________(Ģī”°²»ŹÜÓ°Ļģ”±”¢”°Ę«µĶ”±»ņ”°Ę«øß”±)£¬ŌŅņŹĒ___________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(NH4)2Fe(SO4)2·6H2OĖ×ĆūĦ¶ūŃĪ£¬¼Ūøń±ćŅĖ£¬æÉÓĆĄ“¾»Ė®»ņÖĪĮĘȱĢśŠŌʶŃŖµČ£¬ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£
£Ø1£©Ä¦¶ūŃĪµÄĖ®ČÜŅŗ³Ź”””””””””””””” É«”£
£Ø2£©c(Fe2+)ĻąĶ¬µÄĦ¶ūŃĪŗĶĮņĖįŃĒĢśĮ½ČÜŅŗ±Č½Ļ£¬ĪļÖŹµÄĮæÅØ¶Č½Ļ“óµÄŹĒ”””””””””””” ”£
£Ø3£©¼×ŅŅĮ½Ī»Ķ¬Ń§Ļė²ā¶ØŅ»ĘæĦ¶ūŃĪµÄ“æ¶Č”£¼×Ķ¬Ń§Éč¼ĘµÄŹµŃé×°ÖĆČēĻĀĶ¼£ŗ
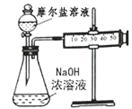
“ÓÉĻĶ¼ĶʶĻ¼×Ķ¬Ń§ĻėĶعż²ā¶Ø”””” ¼ĘĖćĦ¶ūŃĪµÄ“æ¶Č”£øĆ·½°øµÄ²»×ćÖ®“¦ŌŚÓŚ”””””””””””£
£Ø4£©ŅŅĶ¬Ń§ŌŚ¼×Ķ¬Ń§µÄ»ł“”ÉĻ×öĮĖøĽų£¬Ę䏵Ńé×°ÖĆČēĻĀĶ¼£ŗ
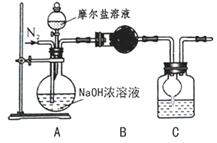
ŌņBÓėCÖŠµÄŹŌ¼Į·Ö±šŹĒ”” ””””””””””””””””””””””ŗĶ”””””””” ”””””””””””””””””£CÖŠøÉŌļ¹ÜµÄ×÷ÓĆŹĒ”””””””””””””” ”””””””””””””””””””””””””””£ŅŅĶ¬Ń§³ĘČ”ĮĖ 10.0gµÄĦ¶ūŃĪѳʷ£¬ČōŹµŃéĶź±Ļŗó²āµĆĻ“ĘųĘæ¼°ĘæÄŚČÜŅŗ¹²ŌöÖŲ 0.68g£¬ŌņŅĄ“ĖŹż¾ŻĶĘĖćÕā·ŻÄ¦¶ūŃĪµÄ“æ¶ČĪŖ”” ””””%£Ø“š°ø¾«Č·µ½Š”ŹżµćŗóµŚŅ»Ī»£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ: £¬“ÓA³ö·¢·¢ÉśĶ¼Ź¾ÖŠµÄŅ»ĻµĮŠ·“Ó¦£¬ĘäÖŠBŗĶC°“1:2·“Ӧɜ³ÉZ,FŗĶE°“1:2·“Ӧɜ³ÉW£¬WŗĶZ»„ĪŖĶ¬·ÖŅģ¹¹Ģ唣
£¬“ÓA³ö·¢·¢ÉśĶ¼Ź¾ÖŠµÄŅ»ĻµĮŠ·“Ó¦£¬ĘäÖŠBŗĶC°“1:2·“Ӧɜ³ÉZ,FŗĶE°“1:2·“Ӧɜ³ÉW£¬WŗĶZ»„ĪŖĶ¬·ÖŅģ¹¹Ģ唣
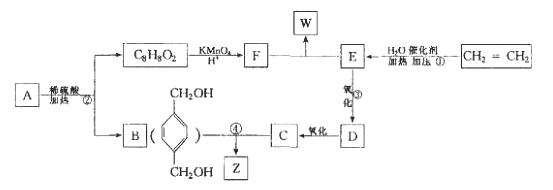
»Ų“šĻĀĮŠĪŹĢā:
(1)Š“³ö·“Ó¦ĄąŠĶ:¢Ł_____________________£¬¢Ś______________________________”£
(2)Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½:
¢Ū_____________________________________________________£»
¢Ü_____________________________________________________”£
(3)ÓėB»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬ŹōÓŚ·ÓĄąĒŅ±½»·ÉĻÖ»ÓŠĮ½øö»„ĪŖ¶ŌĪ»Č”“ś»łµÄ»ÆŗĻĪļÓŠ4ÖÖ£¬Ęä½į¹¹¼ņŹ½ĪŖ_______________£¬_________________£¬_______________£¬______________”£
£Ø4£©AµÄ½į¹¹¼ņŹ½æÉÄÜĪŖ_______________________________________(Ö»Š“Ņ»ÖÖ¼“æÉ)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°ŃÓÉNaOH”¢AlCl3”¢MgCl2ČżÖÖ¹ĢĢå×é³ÉµÄ»ģŗĻĪļ£¬ČÜÓŚ×ćĮæĖ®ÖŠŗóÓŠ0.58 g°×É«³ĮµķÉś³É£¬ŌŚĖłµĆµÄ»ė×ĒŅŗÖŠ£¬ÖšµĪ¼ÓČė0.5 mol/LŃĪĖį£¬¼ÓČėŃĪĖįµÄĢå»ż(V)ÓėÉś³É³ĮµķµÄÖŹĮæ(W)¹ŲĻµČēĶ¼ĖłŹ¾£ŗ
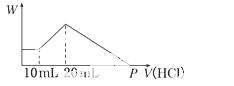
£Ø1£©»ģŗĻĪļÖŠNaOHµÄÖŹĮæĪŖ””””””””””£¬»ģŗĻĪļÖŠAlCl3µÄÖŹĮæĪŖ””””””””””””£¬»ģŗĻĪļÖŠMgCl2µÄÖŹĮæĪŖ””””””””””””.
£Ø2£©Pµć±ķŹ¾ŃĪĖįµÄĢå»żŹĒ”””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»Ų“šŹµŃéŹŅÅäÖĘ0.1 mol/LµÄNaOHČÜŅŗ480 mLŹµŃéÖŠµÄĻĀĮŠĪŹĢā”£
(1)ŠčNaOH_________g”£
(2)ÓŠŅŌĻĀŅĒĘ÷£ŗ¢ŁÉÕ± ¢Ś100 mLĮæĶ² ¢Ū1000 mLČŻĮæĘæ ¢Ü500 mLČŻĮæĘæ
¢Ż²£Į§°ō ¢ŽĶŠÅĢĢģĘ½”£ ÅäÖĘŹ±£¬±ŲŠėŹ¹ÓƵÄŅĒĘ÷ÓŠ____________________£¬
»¹Č±ÉŁµÄŅĒĘ÷ŹĒ__________________”£
(3)ĻĀĮŠ³ĘĮæNaOHµÄ²Ł×÷ÖŠ£¬ÕżČ·µÄŹĒ_______________(ĢīŠņŗÅ£¬ĻĀĶ¬)
¢Ł°ŃNaOHÖ±½Ó·ÅŌŚĶŠÅĢÉĻ³ĘĮæ ¢Ś°ŃNaOH·ÅŌŚÖ½ÉĻ³ĘĮæ
¢Ū°ŃNaOH·ÅČėÉÕ±ÖŠ³ĘĮæ
(4)Ź¹ÓĆČŻĮæĘæĒ°±ŲŠė½ųŠŠµÄŅ»²½²Ł×÷ŹĒ_______________________________”£
(5)ÅäÖĘ¹ż³ĢÖŠ£¬ĻĀĮŠ²Ł×÷»įŅżĘšÅضČĘ«øߵďĒ_______________________”£
¢ŁĪ“Ļ“µÓÉÕ±”¢²£Į§°ō ¢ŚNaOHČÜŅŗĪ“ĄäČ“ÖĮŹŅĪĀ¾Ķ×ŖŅʵ½ČŻĮæĘæÖŠ
¢ŪČŻĮæĘæ²»øÉŌļ£¬ŗ¬ÓŠÉŁĮæÕōĮóĖ® ¢Ü³ĘĮæNaOHµÄŹ±¼äĢ«³¤
¢Ż¶ØČŻŹ±ø©ŹÓæĢ¶Č
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com