
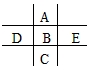
��ͼΪ���ڱ���һС���֣�A��B��C��D��E��F��λ�ù�ϵ��ͼ��ʾ������BԪ�ص�����Ǹ��۾���ֵ��3������������������к���60�����ش��������⣺
F A
D B E
C
��1��A�����ڱ��е�λ��Ϊ ��E�����ӽṹʾ��ͼΪ ��������DE3�ĵ���ʽ_______��
��2��FԪ���⻯��Ŀռ乹��Ϊ_______��
��3��D��B��EԪ�ص�����������Ӧˮ������ᣨ����ǿ��˳�� �����û�ѧʽ��ʾ��
��4��B��C��E����̬�⻯���ȶ�����ǿ������˳����________�����û�ѧʽ��ʾ��
��5��B�ĵ�����������������ˮ�����Ũ��Һ�����ܷ�����Ӧ����ѧ����ʽΪ
��
��6��F2H4��FO2��һ��˫��ֻ���ƽ������������ʻ�Ϸ�����Ӧ����F2��H2O��g������֪8g F2H4������������Ӧ�зų�142kJ���������Ȼ�ѧ����ʽΪ ��
��7�����ڴ������Ӧλ�û���������ǽ���Ԫ�صķֽ��ߡ�
��18�֣���1�� �ڶ����ڢ�A�飻 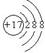 ������ʽ
������ʽ 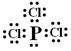 ��
��
��2�������� ��3��HClO4>H2SO4>H3PO4 ��4��HCl>H2S>H2Se
��5��S+2H2SO4��Ũ��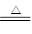 3SO2��+2H2O
3SO2��+2H2O
��6��2N2H4(g)+2NO2(g)��3N2(g)+4H2O(g) ��H����1136 kJ?mol-1
��7�����������ͷǽ����ķֽ��ߡ�
F A
D B E
C
��������
���������BԪ�ص�����Ǹ��۾���ֵ��3��������B�ǵڢ�A��Ԫ�أ�����ǣ�6�ۡ���������������к���60������B�����ԭ��������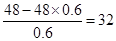 ������B��SԪ�ء���A����Ԫ�أ�F�ǵ�Ԫ�أ�D����Ԫ�أ�E����Ԫ�أ�C��SeԪ�ء��ǽ�����Խǿ������������ˮ���������Խǿ����D��B��EԪ�ص�����������Ӧˮ������ᣨ����ǿ��˳����HClO4>H2SO4>H3PO4���ǽ�����Խǿ���⻯����ȶ���Խǿ����B��C��E����̬�⻯���ȶ�����ǿ������˳����HCl>H2S>H2Se��8g F2H4������������Ӧ�зų�142kJ��������1molN2H4�ų���������
������B��SԪ�ء���A����Ԫ�أ�F�ǵ�Ԫ�أ�D����Ԫ�أ�E����Ԫ�أ�C��SeԪ�ء��ǽ�����Խǿ������������ˮ���������Խǿ����D��B��EԪ�ص�����������Ӧˮ������ᣨ����ǿ��˳����HClO4>H2SO4>H3PO4���ǽ�����Խǿ���⻯����ȶ���Խǿ����B��C��E����̬�⻯���ȶ�����ǿ������˳����HCl>H2S>H2Se��8g F2H4������������Ӧ�зų�142kJ��������1molN2H4�ų���������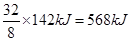 �����Ը÷�Ӧ���Ȼ�ѧ����ʽ��2N2H4(g)+2NO2(g)��3N2(g)+4H2O(g) ��H����1136 kJ?mol-1��
�����Ը÷�Ӧ���Ȼ�ѧ����ʽ��2N2H4(g)+2NO2(g)��3N2(g)+4H2O(g) ��H����1136 kJ?mol-1��
���㣺����Ԫ�����ڱ��ڽṹ��Ԫ�������ɵ�Ӧ���Լ�������ѧ�������д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ�������������ѧ����������������Ӧ��������������Ҫ��Ԫ�ء�λ�������ԡ����߹�ϵ���ۺϿ��飬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ��������������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ���������������������ѧ���������������ͷ�ɢ˼ά�����Լ��淶�Ͻ��Ĵ���������


 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
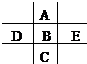 ��ͼΪ���ڱ���һС���֣�A��B��C��D��E��λ�ù�ϵ��ͼ��ʾ������BԪ�ص��������������۾���ֵ��3������������������к���60%���ش��������⣺
��ͼΪ���ڱ���һС���֣�A��B��C��D��E��λ�ù�ϵ��ͼ��ʾ������BԪ�ص��������������۾���ֵ��3������������������к���60%���ش��������⣺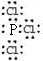
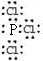
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭�����������ѧУ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��ͼΪ���ڱ���һС���֣�A��B��C��D��E��F��λ�ù�ϵ��ͼ��ʾ������BԪ�ص�����Ǹ��۾���ֵ��3������������������к���60�����ش��������⣺
| | | F | A | |
| | | D | B | E |
| | | | C | |
| | | | | |
| | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0118 ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������1�� ���ʽṹ Ԫ�������ɡ�2011�굥Ԫ���Ծ���һ��������ʡ�ܿ��У��������棩 ���ͣ������
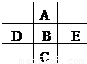 ��ͼΪ���ڱ���һС���֣�A��B��C��D��E��λ�ù�ϵ��ͼ��ʾ������BԪ�ص��������������۾���ֵ��3������������������к���60%���ش��������⣺
��ͼΪ���ڱ���һС���֣�A��B��C��D��E��λ�ù�ϵ��ͼ��ʾ������BԪ�ص��������������۾���ֵ��3������������������к���60%���ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com