
��ij������Һ(���ܺ���Cl����Br����SO42����H2SO3��NH4��)�ֱ��������ʵ�飺
�ټ���ʱ�ų����������ʹƷ����Һ��ɫ��
�ڼӼ�������Ժ���ʱ�ų����������ʹ��ʪ�ĺ�ɫʯ����ֽ������
�ۼ�����ˮʱ����Һ���Ի�ɫ���ټ���BaCl2��Һ�������İ�ɫ����������ϡ���ᣮ
�����������ʲ���ȷ������ԭ��Һ���Ƿ���ڵ���
A��Cl�� B��SO42�� C��H2SO3 D��NH4��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| IO | - 3 |
| S2O | 2- 3 |
| 0.01mol?L-1KIO3 ������Һ�������ۣ� �����/mL |
0.01mol?L-Na2SO3 ��Һ�����/mL |
H2O�� ���/mL |
ʵ�� �¶� /�� |
��Һ���� ��ɫʱ�� ��ʱ��/s | |
| ʵ��1 | 5 | V1 | 35 | 25 | t1 |
| ʵ��2 | 5 | 5 | 40 | 25 | t2 |
| ʵ��3 | 5 | 5 | V2 | 0 | t3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
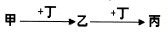
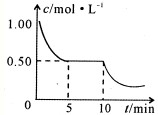
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ�
ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?�㽭ģ�⣩ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ�
��2012?�㽭ģ�⣩ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鷽�� | ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com