
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
���� ����ʽ��HCl ��Է���������36.5,�ܶȣ�1.19 g��cm��3 HCl������������36.5% |
��1����Ũ������HCl�����ʵ���Ũ��Ϊ__ ____mol��L��1��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����________��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl������Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.400 mol��L��1��ϡ���ᡣ
����ѧ����Ҫ��ȡ___ _____mL����Ũ�������������
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿(����������A��ʾ��ƫ��������B��ʾ��ƫС������C��ʾ����Ӱ����)��
a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�� ( )
b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ ( )
��4���������ͬѧ�ɹ�������0.400 mol��L��1�����ᣬ�����ø������кͺ�0.4 g NaOH��NaOH��Һ�����ͬѧ��ȡ________mL���ᡣ����ȷ��С�����һλ��
�������ͬѧ�������Ƶ������кͺ�0.4 g NaOH��NaOH��Һ�����ֱ������������ƫС������ܵ�ԭ����________��
A��Ũ����ӷ���Ũ�Ȳ���
B��������Һʱ��δϴ���ձ�
C��������Һʱ����������ƿ�̶���
D����ˮʱ�����̶��ߣ��ý�ͷ�ι�����
(1)11.9 (2)BD (3)��16.8 ��a.B b��B (4)��25 ��C
��������
�����������1������c��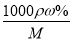 ��֪����Ũ������HCl�����ʵ���Ũ��c��
��֪����Ũ������HCl�����ʵ���Ũ��c��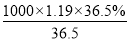 mol/L��11.9mol/L��
mol/L��11.9mol/L��
��2����Һ�Ǿ�һ���ȶ��Ļ�������ȡ����������ĸ�������Һʱ��A������n��cV��֪����Һ��HCl�����ʵ�������Һ������й�ϵ��A����ȷ��B����Һ��Ũ���Dz���ģ�����11.9mol/L��B��ȷ��C����Һ��Cl������Ŀ���Ȼ�������ʵ����й�ϵ���������Һ������й�ϵ��C����ȷ��D����Һ���ܶ�����Һ�����ʣ�����Һ�������ϵ��D��ȷ����ѡBD��
��3���ٸ���ϡ���������ʵ����������֪������500 mL���ʵ���Ũ��Ϊ0.400 mol��L��1��ϡ������Ҫ��Ũ����������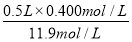 ��0.0168L��16.8ml��
��0.0168L��16.8ml��
������cB��nB/V�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ�����B����Һ�����V����ġ�������ʱ���ؼ�Ҫ�����ƹ�������������V�����ı仯��������һ�����ʵ���Ũ����Һʱ����nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ������Ũ�����ƫС�����������Һ��Ũ��ƫ�ͣ���ѡB��b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ������Һ��������ӣ�����������Һ��Ũ��ƫ�ͣ���ѡB��
��4����0.4 g NaOH�����ʵ�����0.4g��40g/mol��0.01mol������ݷ���ʽHCl��NaOH=NaCl��H2O��֪�������Ȼ�������ʵ�����0.01mol�������Ҫ������������0.01mol��0.400mol/L��0.025L��25ml��
��A��Ũ����ӷ���Ũ�Ȳ����������������Ƶ����ʵ�������������£�����������Һ�����ƫ��A����ȷ��B��������Һʱ��δϴ���ձ������������Ũ��ƫС��������������Ƶ����ʵ�������������£�����������Һ�����ƫ��B����ȷ��C��������Һʱ����������ƿ�̶�����������ƿ����Һ�����ƫС��������������Ƶ����ʵ�������������£�����������Һ�����ƫС��C��ȷ��D����ˮʱ�����̶��ߣ��ý�ͷ�ι����������������Ũ��ƫС��������������Ƶ����ʵ�������������£�����������Һ�����ƫ��D����ȷ����ѡC��
���㣺�������ʵ���Ũ�ȵ��йؼ��㡢���ơ��������Ե�


 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ�����и�һ3���ʼ컯ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ��̽����̽��̼����Ԫ�صķǽ����Ե����ǿ��
����Ҫ��������и�С��
��1��ʵ��װ�ã�
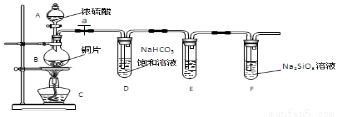
��д��ʾ��������A B
��2��ʵ�鲽�裺
���������� ����ҩƷ��a��Ȼ�����Ũ���ᣬ����
��3������̽��������֪����ǿ��:������ >̼�ᣩ
��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��װ��E����������KMnO4��Һ�������� ��
����˵��̼Ԫ�صķǽ����Աȹ�Ԫ�طǽ�����ǿ��ʵ�������� ��
�������Թ�D�е�ʵ�������ܷ�֤����Ԫ�صķǽ�����ǿ��̼Ԫ�صķǽ����� ����ܡ������Թ�D�з�����Ӧ�����ӷ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ̩���и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�������
��19.2 g Cu����������ϡ�����У�����Cu��ȫ��Ӧ��
������1�����ɵ�NO�ڱ�״���µ������
��2������ԭ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ̩���и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����и������ʰ����ʡ��ᡢ��η���˳�����У�������ȷ����
A�����������ᡢ�������ͭ B����ơ����ᡢ�ռ���ᱵ
C��ˮ�������ᡢ�ռ�������� D���������ᡢʯ��ˮ���Ȼ�ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ̩���и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij���⻯ѧ�̲�����һ�Ź���������ԭ��Ӧ�IJ�ͼ����ͼ��֪���ڸ÷�Ӧ��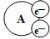 ��
��
A����ԭ�� B�������� C���������� D����ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
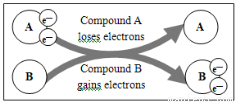
���з�Ӧ����Ԫ�ؽ�����ԭ����
A. 5Cl2��I2��6H2O��10HCl��2HIO3
B. 2Cl2��2Ca(OH)2��CaCl2��Ca(ClO)2��2H2O
C.MnO2��4HCl(Ũ)  MnCl2��2H2O��Cl2��
MnCl2��2H2O��Cl2��
D.HCl��NaOH��NaCl��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A��ͬ��ͬѹ�¼�����CH4�����������ܶ�֮��Ϊ2��1
B��1 g�����1 g������ԭ����֮��Ϊ5��1
C�������ʵ����ļ��������������֮��Ϊ2��1
D���ڱ�״���µ������ļ�������������֮��Ϊ1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ�����и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���и�ѡ������������Ҫ��ȡ�����ʣ������ܹ��õ�����
A��CaCO3 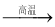 CaO
CaO 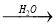 Ca(OH)2
Ca(OH)2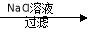 NaOH��Һ
NaOH��Һ
B��Cu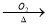 CuO
CuO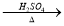 CuSO4��Һ
CuSO4��Һ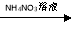 Cu(NO3)2��Һ
Cu(NO3)2��Һ
C��KMnO4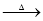 O2
O2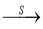 SO3
SO3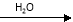 H2SO4
H2SO4
D ��NH3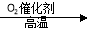 NO
NO NO2
NO2 HNO3
HNO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�켪��ʡ��һ��ѧ������һ����ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ѧ��ȤС�������ʹ������װ����֤��ͭ��һ������Ũ���ᷴӦ��һ���������������������������Ϊ��״����������������������Բ��ƣ��Һ��Է�Ӧ����Һ������仯���Իش��������⣺
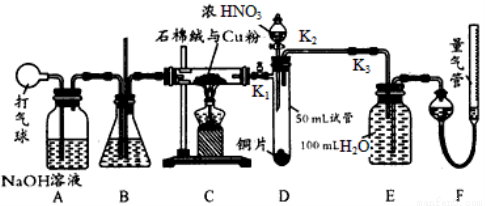
(1)��ͭ��Ũ���ᷴӦǰ����ѹ������A��B��C����װ�ú���װ��D�е������� ���ѧʽ����ͨ��������Ŀ���� �����д˲�����ʱӦ�ر� ������K1����K2������K3������ͬ������ ��
(2)��װ��C��Ӳ�ʲ������г��ֶ�������װ��B�п��ܳ��ֵ������� ��
(3)�ر�K1��K2����K3���ɷ�Һ©����װ��D���Թ��еμ�Ũ���ᡣ��Cu��Ũ���ᷴӦ��������ͨ����Һ©����װ��D���Թ��м���CCl4��������װ��D���Թ���һ�����������ӷ�Ӧ����ʽΪ ��
(4)��װ��E������Һ��ȡ��25��00 mL����0��1000 mol/L��NaOH��Һ�����кͣ���ǡ�ó�����ʱ����NaOH��Һ18��00mL ����װ��E��������������ʵ���Ũ��Ϊ ��
(5)ʵ��ǰ�����ܵ�Һ�����Ϊ368��50 mL��ʵ��������ܵ�Һ�����Ϊ224��00 mL��������ͭ��һ������Ũ���ᷴӦ�� ��������������������NO���ɣ�д���Ƶ����� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com