
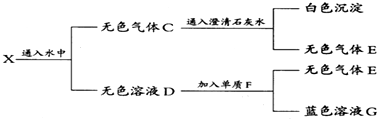
·ÖĪö ĪŽÉ«ĘųĢåCĶØČė³ĪĒåŹÆ»ŅĖ®Éś³É°×É«³Įµķ£¬øĆ³ĮµķÓ¦ĪŖCaCO3£¬ĖµĆ÷CÖŠŗ¬ÓŠCO2£¬ĪŽÉ«Ļ”ČÜŅŗDÓėµ„ÖŹF·“Ӧɜ³ÉĄ¶É«ČÜŅŗ£¬øĆČÜŅŗÓ¦ŗ¬ÓŠCu2+£¬ŌņĖµĆ÷FĪŖCu£¬ĪŽÉ«Ļ”ČÜŅŗD¾ßÓŠĒæŃõ»ÆŠŌ£¬Ó¦ĪŖĻ”HNO3ČÜŅŗ£¬Éś³ÉĘųĢåEĪŖNO£¬ŌņĖµĆ÷»ģŗĻĘųĢåXÓ¦ĪŖCO2ŗĶNO2µÄ»ģŗĻĪļ£¬CĪŖCO2ŗĶNOµÄ»ģŗĻĪļ£¬ŌņAĪŖC£¬BĪŖHNO3£¬½įŗĻĪļÖŹµÄŠŌÖŹ½ā“šøĆĢā£®
½ā“š ½ā£ŗĪŽÉ«ĘųĢåCĶØČė³ĪĒåŹÆ»ŅĖ®Éś³É°×É«³Įµķ£¬øĆ³ĮµķÓ¦ĪŖCaCO3£¬ĖµĆ÷CÖŠŗ¬ÓŠCO2£¬ĪŽÉ«Ļ”ČÜŅŗDÓėµ„ÖŹF·“Ӧɜ³ÉĄ¶É«ČÜŅŗ£¬øĆČÜŅŗÓ¦ŗ¬ÓŠCu2+£¬ŌņĖµĆ÷FĪŖCu£¬ĪŽÉ«Ļ”ČÜŅŗD¾ßÓŠĒæŃõ»ÆŠŌ£¬Ó¦ĪŖĻ”HNO3ČÜŅŗ£¬Éś³ÉĘųĢåEĪŖNO£¬GĪŖCu£ØNO3£©2£¬ŌņĖµĆ÷»ģŗĻĘųĢåXÓ¦ĪŖCO2ŗĶNO2µÄ»ģŗĻĪļ£¬CĪŖCO2ŗĶNOµÄ»ģŗĻĪļ£¬ŌņAĪŖC£¬BĪŖHNO3£¬
¢ŁÓÉÉĻĆęµÄ·ÖĪöæÉÖŖCĪŖCO2ŗĶNO£¬XĪŖCO2ŗĶNO2£¬
¹Ź“š°øĪŖ£ŗCO2ŗĶNO£»CO2ŗĶNO2£»
¢ŚAĪŖC£¬BĪŖHNO3£¬·¢Éś·“Ó¦ĪŖC+4HNO3£ØÅØ£©$\frac{\underline{\;\;”÷\;\;}}{\;}$CO2”ü+4NO2”ü+2H2O£¬
¹Ź“š°øĪŖ£ŗC+4HNO3£ØÅØ£©$\frac{\underline{\;\;”÷\;\;}}{\;}$CO2”ü+4NO2”ü+2H2O£»
¢ŪDĪŖĻ”HNO3ČÜŅŗ£¬FĪŖCu£¬DµÄĻ”ČÜŅŗÓėF·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ3Cu+2NO3-+8H+ØT3Cu2++2NO”ü+4H2O£¬
¹Ź“š°øĪŖ£ŗ3Cu+2NO3-+8H+ØT3Cu2++2NO”ü+4H2O£®
µćĘĄ ±¾Ģāæ¼²éĪŽ»śĪļµÄĶʶĻ£¬ĢāÄæÄŃ¶Č²»“󣬱¾Ģā×¢Ņāøł¾ŻĪļÖŹµÄŠŌÖŹŗĶ·“Ó¦µÄĢŲÕ÷ĻÖĻó£¬Č·¶ØGŗĶCO2£¬½įŗĻĪļÖŹµÄŠŌÖŹÓĆÄęĶʵķ½·ØĶʶĻ£¬æÉŅĄ“ĪĶʶĻĘäĖüĪļÖŹ£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 8.0g | B£® | 11.7g | C£® | 23.4g | D£® | ĪŽ·Ø¼ĘĖć |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2.16g | B£® | 1.08g | C£® | 2.7g | D£® | 5.4g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĪĀ¶ČŌ½øߣ¬KÖµŌ½“ó | B£® | KÖµŌ½“ó£¬Õż·“Ó¦ĖŁĀŹŌ½“ó | ||
| C£® | KÖµµÄ“óŠ”ÓėĘšŹ¼ÅضČÓŠ¹Ų | D£® | KÖµŌ½“󣬷“Ó¦ĪļµÄ×Ŗ»ÆĀŹŌ½“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÅØĻõĖį±£“ęŌŚ×ŲÉ«ŹŌ¼ĮĘæÖŠ | B£® | ÉŁĮ潚ŹōÄĘæɱ£“ęŌŚĆŗÓĶĄļ | ||
| C£® | ŠĀÖĘĀČĖ®±£“ęŌŚ×ŲÉ«ŹŌ¼ĮĘæÖŠ | D£® | ÅØĮņĖį±£“ęŌŚĻš½ŗČūŹŌ¼ĮĘæÖŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Al3+ŗĶAl£ØOH£©3 | B£® | Al£ØOH£©3 | C£® | AlO2- | D£® | AlO2-ŗĶAl£ØOH£©3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com