
(��9�֣���֪NaAlO2��HCl��H2O��Al(OH)3����NaCl��ij�����A����
KAl(SO4)2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
 �ݴ˻ش��������⣺
�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����� ��
��2������������ͼ��Ӧ��ϵ��д������B��D�������ʵĻ�ѧʽ
����B ������D ��
��3��д���١��ڡ����ĸ���Ӧ�Ļ�ѧ����ʽ�������ӷ�Ӧ��д�����ӷ���ʽ
�� ��
�� ��
�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����10�֣���֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����Ӧ����ʽΪ2NH3��3CuO
![]() N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ���ش��������⣺
N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ���ش��������⣺
(1��A�з�����Ӧ�Ļ�ѧ����ʽ�� �����鰱��ͨ�����õķ�����������_________ ____________�� ��
(2��B���������� ���������� ��
(3��ʵ��ʱC�й۲쵽�������� ���÷�Ӧ�а���������_______����
(4����Ҫ���鷴Ӧ���ɵ�ˮ���ɽ��Թ�D���ձ����ָij����������ĸ���ܣ���һ�������X��װ��_____________��������___________________���ڶ��������Y��װ�м�ʯ�ң�������____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
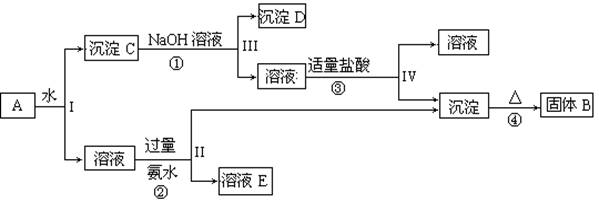
(��9�֣���֪NaAlO2��HCl��H2O��Al(OH)3����NaCl��ij�����A����
KAl(SO4)2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
 �ݴ˻ش��������⣺
�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����� ��
��2������������ͼ��Ӧ��ϵ��д������B��D�������ʵĻ�ѧʽ
����B ������D ��
��3��д���١��ڡ����ĸ���Ӧ�Ļ�ѧ����ʽ�������ӷ�Ӧ��д�����ӷ���ʽ
�� ��
�� ��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��Ϫһ�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
(��9�֣���֪NaAlO2��HCl��H2O��Al(OH)3����NaCl��ij�����A����
KAl(SO4)2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��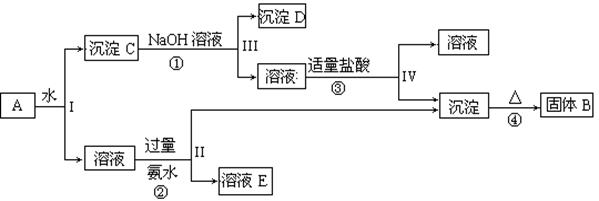 �ݴ˻ش��������⣺
�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����� ��
��2������������ͼ��Ӧ��ϵ��д������B��D�������ʵĻ�ѧʽ
����B ������D ��
��3��д���١��ڡ����ĸ���Ӧ�Ļ�ѧ����ʽ�������ӷ�Ӧ��д�����ӷ���ʽ
�� ��
�� ��
��  ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
��ÿ��1�֣���10�֣���֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��������ԭ�Ӳ�ȡ �ӻ���
��3��д��������AC2�ĵ���ʽ ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ����һ��ֻ��B��ɵ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ��
��4��E�ĺ�������Ų�ʽ�� ��ECl3�γɵ������Ļ�ѧʽΪ ��
��5��D���ʾ���ѻ�Ϊ____________________���á��ʾDԭ�ӣ��ڷ����ڻ����侧��ͼ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com