
��ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�
��֪��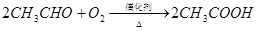
��1����ӦII�Ļ�ѧ����ʽ�� ��
��2��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ�� ��
��3��E������ζ�����ʣ���ʵ��������ͼװ����ȡ��
�ٷ�ӦIV�Ļ�ѧ����ʽ�� ���÷�Ӧ����Ϊ ��
�ڸ�װ��ͼ����һ�����ԵĴ����� ��
��4��Ϊ��֤��Ũ�����ڷ�ӦIV�����˴�������ˮ�������ã�ijͬѧ������ͼ�Ľ���װ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ����ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܼ����Լ� | �Թ������Լ� | �л���ĺ��/cm |
| A | 2 mL�Ҵ���1 mL���ᡢ 1mL18mol��L��1Ũ���� | ����Na2CO3��Һ | 3.0 |
| B | 2 mL�Ҵ���1 mL���� | 0.1 | |
| C | 2 mL�Ҵ���1 mL���ᡢ 3 mL 2mol��L��1 H2SO4 | 0.6 | |
| D | 2 mL�Ҵ���1 mL���ᡢ���� | 0.6 |
��1��2CH3CH2OH��O2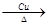 2CH3CHO��2H2O��2�֣�����Ҳ��д��
2CH3CHO��2H2O��2�֣�����Ҳ��д�� ��
��
��2��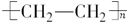 ��1�֣�
��1�֣�
��3����CH3COOH��HOCH2CH3 CH3COOCH2CH3��H2O����2�֣���Ũ���ᡢ���ȡ���
CH3COOCH2CH3��H2O����2�֣���Ũ���ᡢ���ȡ��� ��ȱһ���1�֣� ȡ����Ӧ��������Ӧ��1�֣�
��ȱһ���1�֣� ȡ����Ӧ��������Ӧ��1�֣�
�ڵ����ܵij������뵽����̼������ҺҺ�����£�2�֣�
��4���� 4 ��1�֣� ��A C��2��,��ѡ���÷֣�©ѡ��1�֣�
���������������ϩ����̼̼˫�����ܷ����Ӿ۷�Ӧ�����ɾ���ϩ����D�Ǿ���ϩ����ϩҲ�ܺ�ˮ�����ӳɷ�Ӧ�����Ҵ�����A���Ҵ����Ҵ�����������Ӧ����B������B����ȩ����ȩ�����������������ᣬ����C�����ᡣ������Ҵ�����������Ӧ����������������E�����ᡣ
��1����ӦII���Ҵ��Ĵ�����������ȩ����Ӧ�Ļ�ѧ����ʽ��2CH3CH2OH��O2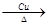 2CH3CHO��2H2O��
2CH3CHO��2H2O��
��2��DΪ����ϩ�����ڸ߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ��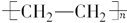 ��
��
��3���ٷ�ӦIV���Ʊ����������ģ���Ӧ�Ļ�ѧ����ʽ��
CH3COOH��HOCH2CH3 CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��
���������ɵ����������к���������Ҵ������߶�����ˮ���ܵģ�����װ�������ֱ�Ӳ��뵽̼������Һ�У����������������Ը�װ��ͼ����һ�����ԵĴ����ǵ����ܵij������뵽����̼������ҺҺ�����¡�
��4����ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����ã�������ʵ���������ӵ�Ũ��Ӧ������ͬ�ġ�C�������Ƕ�Ԫǿ�ᣬ���Ը��������Ũ�ȿ�֪��D�������Ũ��Ӧ����4mol/L��
�ڸ��ݱ������ݿ�֪��ʵ��A��C�зֱ������Ũ�����ϡ���ᣬ����ͨ������ʵ��A��C�����ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʡ�
���㣺���⿼�鿼���л�����ƶϡ��л���Ӧ���͡�����ʽ����д�Լ�ʵ�鷽�����������ۡ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Тټ��飬�����飬�۱��飬�ܶ���4���������Իش��������⣺
��1����д����������ͨʽ��ʾ���������������г��ȼ�յĻ�ѧ����ʽ��
________________________________________________________________________��
��2����ͬ״���£��������������̬��������O2����������________ ��
��3����������������̬�����ڳ��ȼ��ʱ������O2����������________ ��
��4������ͬ���칹�����_______ _��
��5����120 �棬1.01��105Pa�����£�ij��̬����������O2��ȫ��Ӧ��÷�Ӧǰ����������û�з����ı䣬�����Ϊ________ ��
��6��10 mLij��̬������50 mL O2�г��ȼ�գ��õ�Һ̬ˮ���Լ����Ϊ35 mL�Ļ������(���������������ͬ��ͬѹ�²ⶨ)������̬����________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij�����л������Է�������Ϊ60��1 mol���л�����ȫȼ�գ�����36gH2O��44.8L CO2����״���£���
��1������л������ʽ��
��2����֪���л�����������ԣ���������Ʒ�Ӧ��Ҳ�������Һ��Ӧ����д�������ܵĽṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С����ȼ�շ������ⶨij�л�����̼�����Ԫ�صĺ���������ֶ������������̽�������ѳ�������Ʒ�����������У�������ͭ���������ڸ�����������Ʒȫ��������Ϊˮ�Ͷ�����̼��Ȼ��ֱ�ⶨ���ɵ�ˮ�Ͷ�����̼��ʵ������õ���װ������ͼ��ʾ������Aװ�ÿ����ظ�ʹ�á�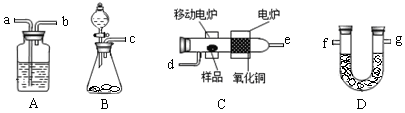
��ش��������⣺
��1���밴������������ʵ��װ�� �� �� ��d�� �� �� �� g���������ӿڱ����д����
��2��Bװ������O2ʱ���õ�ҩƷ�� ��ʵ���У���ʼ��Cװ�ü���֮ǰ��Ҫͨһ��ʱ���������Ŀ���� ��ֹͣ���Ⱥ�ҲҪ��ͨһ��ʱ���������Ŀ���� ��
��3����֪ȡ2��3g����ƷX��������ʵ�飬���ⶨAװ������2��7g��Dװ������4��4g���������X���ʵ�ʵ��ʽ �� ��4����С��ͬѧ��һ��ʵ���ã�2��3g�� X����������Ʒ�Ӧ�ɷų�560mLH2���ѻ���ɱ�״���£�������֪X����ֻ��һ�������š��������Ϻ�ѧ�����ֽ���������̽��ʵ�飺
��4����С��ͬѧ��һ��ʵ���ã�2��3g�� X����������Ʒ�Ӧ�ɷų�560mLH2���ѻ���ɱ�״���£�������֪X����ֻ��һ�������š��������Ϻ�ѧ�����ֽ���������̽��ʵ�飺
ʵ��һ��X��һ�������¿ɴ��������������л���Y��
ʵ�����X��Y��Ũ������������������л���Z��
���д��ʵ����з�Ӧ�Ļ�ѧ����ʽ ��
�ڳ�ȥZ�л��е�Y������Լ�����Ҫ������ �� ��
��5������֪������2��3gҺ̬X����������ȫȼ�����ɶ�����̼�����Һ̬ˮʱ�ɷų�68��35kJ��������д��X��������ȼ�յ��Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ѧ�ϳ���ȼ�շ�ȷ���л�����ɣ����ַ������ڵ�¯����ʱ�ô���������������Ʒ�����ݲ��������ȷ���л������ɡ��л���M(����ʽ��CxHySz)�����ηɻ����������ϵ���Ҫ�ɷ֡�
M���ȼ�յIJ���Ϊ���������д����ѧ����ʽ��_______ _______��
����ʵ�飺
ij��ѧ��ȤС��Ϊ��֤M���Ԫ�ؽ���������ʵ��:��������Ʒ����ȼ�չ�A�У�ͨ��һ����O2���õ�¯����ʹ��ȼ��,����װ����ͼ��ʾ(�г�������װ������ȥ)��
��1����ʵ��װ������˳��Ϊ____________________��������ÿһ������ֻ��ʹ��һ�Σ�
��2��D��ʢ�ŵ��Լ���________
��3����֤���л��ﺬ̼Ԫ�ص�������_________________________________________��
��4��ȼ�չ��з���CuO��������________________________________��
��5��ָ�������д����װ�ã�__________________________________________________��
����ʵ�飺
��1���������CO2���������������ͼ��ʾװ�ã�ʵ�������ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ������ȴ�����£��۶�ȡ��Ͳ��������������������������ȷ˳���ǣ�________������д������ţ���
��2���������CO2�������������ó����������г�������õ���
a��0.1mol/LCaCl2��Һ b��0.1mol/L Ca(OH)2��Һ
c��0.1mol/L Ba(NO3)2��Һ d��0.1mol/L Ba(OH)2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1�����Ǿ�����ȼ���Ҵ�����������ȷ���Ҵ��к���C��H����Ԫ�ء���Ҫ˵�����ǵľ����������֤��������Ԫ�صIJ�����________________________________________________________ _
________________________________________________________________________________________��
��֤������̼Ԫ�صIJ�����________________________________________________
_______________________________________________________________________
��2��Ҫ��ȼ�շ�������֤ʵ�Ҵ��л�������Ԫ��ʱ����ȡ��һЩʵ�����ݣ���Щ����Ӧ����________________________��
��3��Ϊȷ���Ҵ��ķ���ʽ������2���������⣬���費��Ҫ�ⶨ�Ҵ�����Է�������?
_______________________________________________________________________
��4��Ϊ�ⶨ�Ҵ����ӽṹ������������ˮ�Ҵ��ͽ����Ʒ�Ӧ�ռ����������ķ�����ѡ��������ͼ��ʾ������(�е���������˫����Ƥ��)��
��װ�õ�����˳����______��______��______��______��_______��_______��
����֪��ˮ�ƾ����ܶ�Ϊ0��789 g��cmһ3����ȡ2��0mL�ƾ�����Ӧ��ȫ��(�ƹ���)���ռ�390 mL���塣���Ҵ��������ܱ���ȡ��������ԭ����Ϊ_______���ɴ˿�ȷ���Ҵ��ĽṹΪ______________________������______________��
��5��ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����(��д���)��______________
A����ʵ���������½���
B����ˮ�ƾ��л������״�
C����ˮ�ƾ����Ʒ�Ӧ������ȫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪����������ˮ�е��ܽ��Ϊ��0.18g��4�棩��0.34g��25�棩��6.8g��95�棩�����ѵķе�Ϊ34.6�档ʵ���ҳ��ñ���ȩ�Ʊ����״��ͱ����ᣬ��ԭ��Ϊ��2C6H5�DCHO��NaOH C6H5�DCH2OH��C6H5�DCOONa
C6H5�DCH2OH��C6H5�DCOONa
ʵ�鲽�����£�
������ͼ��ʾװ���м������� NaOH��ˮ�ͱ���ȩ�����ȡ����ȣ�ʹ��Ӧ��ֽ��С�
�ڴ��������¿ڼ�����ˮ�����ȣ���ȴ�������Һ©������������ȡ����Һ��ˮ�㱣�����á������Ѳ�������10%̼������Һ��ˮϴ�ӡ�
�۽����Ѳ㵹��ʢ��������ˮ����þ�ĸ�����ƿ�У����ȡ����ú���ת������װ�ã��������ȼ��ȳ�ȥ���ѣ��ռ�198�桫204����ֵñ��״���
�ܽ�������е�ˮ�������Ũ�����Ͼ��ȣ�������ɫ���塣��ȴ�����˵ôֲ�Ʒ�����ֲ�Ʒ�ᴿ�ñ����ᡣ
��1��������У������ˮϴ�ӵ������� ������Һ©��������Һ����뿪��ʵ����������ǣ��� �� ��
��2�����������ˮ����þ�������� ��
��3���������ˮ���Ũ�����Ϻ�����Ӧ�Ļ�ѧ����ʽΪ ������Ӧ��������ȴ��Ŀ���� ��
��4������װ������������������ѹϵͳ�⣬���� �� �����������ƣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������У���һ���������ܷ���ȡ����Ӧ�ͼӳɷ�Ӧ��������ʹ���Ը��������Һ��ɫ����
| A������ | B������ | C���� | D����ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
2014��4��10�գ����ݷ�������ˮ�����������¼�������ʯ�ͻ����Ļ���ԭ�ϣ������������ڣ�������Ѫ��֯�����γ���ѪҺ���ԵĴ�л���������������֯�϶�Ϊ�°����ʡ����й��ڱ���������ȷ����
| A�������ǵ���˫��������ɵ�ƽ�滷״�ṹ |
| B��������ϩ����ʹ����KMnO4��Һ��ɫ |
C������������Ӧ������ȡ����Ӧ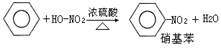 |
| D��������ˮ�ڴ��������·���ȡ����Ӧ�����屽 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com