
A��B��C��D��EΪԭ������������������ֶ�����Ԫ��,���н�����һ�ֽ���Ԫ��,A��D������������ͬ;B��C��E�����ڱ�������,��C��Eͬ���塣B��C������������֮�͵���D��ԭ�Ӻ��������,A��C���γ����ֳ�����Һ̬����� ��ش���������:
(1)B��ԭ�ӽṹʾ��ͼ ��
(2)C��D��E����ԭ�Ӷ�Ӧ�����Ӱ뾶�ɴ�С��˳���� (���������);��A��B��C����Ԫ�ذ�4��2��3��ɵĻ����������Ļ�ѧ������Ϊ ��
(3)��ij�ַ����Ľ�����������A��C��D��ɵĻ�������Һ��Ӧ,�÷�Ӧ�����ӷ���ʽΪ: ��
(4)��100 mL 18 mol/L��Ũ��A��C��E��ɵ�����Һ�м��������ͭƬ,����ʹ֮��ַ�Ӧ, �����������ڱ�״���µ���������� (�����);
a.7.32 L b.6.72 L c.20.16 L d.30.24 L
��ʹ������Ӧ��ʣ���ͭƬ�����ܽ�,�������м���������,��Ӧ�����ӷ���ʽΪ: ��
(5)A��C��Ԫ�صĵ���������K2CO3��ɵ�ȼ�ϵ��,�为����ӦʽΪ ,�øõ�ص��1 L 1 mol/L NaCl��Һ�����ı�״����1.12 L H2ʱ,NaCl��Һ��pH= (�������������Һ���������)��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʡ���������ƵƵ������Ϊ��ֹ�ڴ���֮���߲����У�������Ҫ�����ĸ�����������Ư���ȡ�
(1)����������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��SO2��H2SO4�����·�Ӧ�Ƶá���д����Ӧ�����ӷ���ʽ�� ��
(2)��̼������һ������̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ���̼�������������ʾ��ᷢ����ѧ��Ӧ��ʧЧ�����й�̼����ֻ������������Ӧ���� ��
| A��MnO2 | B��KMnO4��Һ | C��ϡ���� | D��Na2SO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO��Cr2O3���Լ�SiO2��Al2O3�����ʣ�Ϊԭ��������ʵ����ģ�ҵ���ø�������K2Cr2O7����Ҫ�������£�
��Ӧ������Ҫ�����ķ�ӦΪ��
��.FeO��Cr2O3��NaOH��KClO3��Na2CrO4��Fe2O3��H2O+KCl��δ��ƽ��
��.Na2CO3+SiO2�� Na2SiO3+CO2��
��.Al2O3+2NaOH�� 2NaAlO2+H2O
�ڲ�����н���ҺpH���ڵ�7~8���Խ�SiO32-��AlO2-ת��Ϊ��Ӧ�ij�����ȥ��
��1���ڷ�Ӧ������������________������245g KClO3�μӷ�Ӧ����ת�Ƶĵ�����Ϊ_____________��
��2����Ӧ�������ɵ�Fe2O3�ֿɺ�Na2CO3��Ӧ�õ�һ��Ħ������Ϊ111g/mol�Ļ������ǿ��ˮ�⣬�ڲ��������ɳ�������ȥ��д�����ɸû�����Ļ�ѧ��Ӧ����ʽ_____________________________
___________________________��
��3��������Ŀ���ǽ�CrO42-ת��ΪCr2O72-��������Ϊ__________________________�����ӷ���ʽΪ_______________________________________��
��4����ѡ�ú��ʵķ�����һ���ᴿ�ֲ�Ʒ�ظ����__________������ĸ��
A���ؽᾧ B����ȡ��Һ C������
��5��������Ʒ��K2Cr2O7�Ĵ��������������ữ��K2Cr2O7��KI������I2,Ȼ������������ʲ��I2�����Ӷ����K2Cr2O7������д���ữ��K2Cr2O7��KI��Ӧ�Ļ�ѧ����ʽ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ұ���봦�����漰������ԭ��Ӧ��
��1������������ұ����Ӧ����ʱ���õ�ⷨ����________��
a��Fe2O3 b��NaCl c��Cu2S d��Al2O3
��2����ͭ��(Cu2S)�ɷ�����Ӧ��2Cu2S��2H2SO4��5O2=4CuSO4��2H2O���÷�Ӧ�Ļ�ԭ����________����1 mol O2������Ӧʱ����ԭ����ʧ���ӵ����ʵ���Ϊ________mol����CuSO4��Һ�м���þ��ʱ���������ɣ���������________��
��3����ͼΪ��⾫������ʾ��ͼ��________(�a����b��)��Ϊ�������ʵĴ�������b������������ɫ��������������ɸ�����ĵ缫��ӦʽΪ__________________________��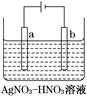
��4��Ϊ������������ĺڰ�(Ag2S)�����������������������ʳ��ˮ�в������Ӵ���Ag2Sת��ΪAg��ʳ��ˮ��������______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����г������ؽ�����Ⱦ���У�����Ǧ���̡������ӡ�������ҵ��ˮ�к��е�Cr2O72-��CrO42-�����õķ��������֡�
����1����ԭ������
�÷��Ĺ�������Ϊ ��
��
���еڢٲ�����ƽ��2CrO42-����ɫ����2H�� Cr2O72-����ɫ����H2O��
Cr2O72-����ɫ����H2O��
��1��д���ڢٲ���Ӧ��ƽ�ⳣ������ʽ_________________________________��
��2�����ڵڢٲ���Ӧ������˵����ȷ����________��
A��ͨ���ⶨ��Һ��pH�����жϷ�Ӧ�Ƿ��Ѵ�ƽ��״̬
B���÷�ӦΪ������ԭ��Ӧ
C��ǿ���Ի�������Һ����ɫΪ��ɫ
��3���ڢڲ��У���ԭ0.1 mol Cr2O72-����Ҫ________mol��FeSO4��7H2O��
��4���ڢ۲�������Cr��OH��3�⣬���������ɵij���Ϊ________������Һ�д������³����ܽ�ƽ�⣺Cr��OH��3��s�� Cr3����aq����3OH����aq���������£�Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
Cr3����aq����3OH����aq���������£�Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
����2����ⷨ
��5��ʵ����������ͼװ��ģ���ⷨ������Cr2O72-�ķ�ˮ�����ʱ������ӦʽΪ________��������ӦʽΪ________���õ��Ľ������������������ɳ�����ȫ����ˮ�ĵ���ƽ��ǶȽ�����ԭ����___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����̼���̿�Ϊ��Ҫԭ������MnO2�Ĺ����������£�
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Pb��OH��2 | Mn��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������[��NH4��2Ce��NO3��6]�㷺Ӧ���ڵ��ӡ�����ҵ����ϳ�·�����£�
��1����֪��NH4��2Ce��NO3��6�����ֽ⣬ij����С����Ϊ��Ӧԭ�����£��벹����������NH4��2Ce��NO3��6 CeO2��8OH + 8_____����CeO2��8OH
CeO2��8OH + 8_____����CeO2��8OH CeO2+ 4H2O��+2O2����
CeO2+ 4H2O��+2O2����
�ڿ����м��ȣ�NH4��2Ce��NO3��6����������ɫ�б仯�⣬���ɹ۲쵽��������_________��
��2��������У���Ce��NO3��3��6H2O�����Һ������pH��4~5����������H2O2��Һ�����Ͼ��ȣ��ټ��백ˮ������ҺpH���õ�Ce��OH��4�������ù����вμӷ�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ___________��
��3��298Kʱ��Ksp[Ce��OH��4]��1��10��29��Ce��OH��4���ܶȻ�����ʽΪKsp��___________��
Ϊ��ʹ��Һ��Ce4��������ȫ������������Һ�е�c��Ce4+��С��1��10��5mol��L��1�������pHΪ______���ϡ�
��4��Ϊ���о������Ĺ�������������С��ⶨ�ˣ�NH4��2Ce��NO3��6�ڲ�ͬ�¶ȡ���ͬŨ�������е��ܽ�ȣ������ͼ����ͼ�пɵó�������Ҫ���ɣ�
�� ��NH4��2Ce��NO3��6�������е��ܽ�����¶����߶�����
�� _____________________________________________��
�� _____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о����ʼ��������ԭ��Ӧ����Ҫ�����塣
��1��һ��������Fe��OH��3��KClO��KOH��Һ�з�Ӧ���Ƶ�K2FeO4�����з�Ӧ����������___________������0��5molK2FeO4ת�Ƶ��ӵ����ʵ���____________mol��
��2�����������һ�����Ͷ��ε�أ����ҺΪ����Һ���䷴ӦʽΪ��
3Zn��OH��2+2Fe��OH��3+4KOH 3Zn+2K2FeO4+8H2O
3Zn+2K2FeO4+8H2O
�ŵ�ʱ����صĸ�����ӦʽΪ____________�����ʱ���Һ��pH____________����������䡱��С����
��3��H2O2��һ����ɫ����������ҵ�Ʊ�H2O2��ԭ�����£�
�ٵ����ܷ�Ӧ��2S2O42?+2H+�TS2O82-+H2��
�ڵ�����ɵ�S2O82-ˮ�⣺S2O82-+2H2O�TH2O2+2H++SO42?
������������Ӧʽ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���б仯�����������úͻ�ԭ���õ�Ԫ�ز���ͬһ��Ԫ�ص���( )
| A����400 ���������д������ڵ������£��ð�����һ��������ԭΪ���� |
| B����ҵ����ϡ����������뵥��ͭ��Ӧ��ȡ����ͭ |
| C����ҵ����ʯ����������Ʊ�Ư�� |
| D��ʵ����������غ�Ũ������ȡ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com