
�ݱ�������һ�ֽ�Thibacillus Ferroxidans��ϸ�������������µ�������Һ�У��ܽ���ͭ��(CuFeS2)�����������Σ������ķ�ӦΪ��
4CuFeS2��2H2SO4��17O2===4CuSO4��2Fe2(SO4)3��2H2O
(1)CuFeS2��Fe�Ļ��ϼ�Ϊ��2��������Ӧ�б�������Ԫ����________��
(2)��ҵ����������������Ӧ�����Һ�����������̿��Ʊ�����(CuSO4��5H2O)��

�ٷ������б���(����Ksp����Ӧ������������ij����ܽ�ƽ�ⳣ��)��
| Ksp | �������↑ʼ ����ʱ��pH | ����������� ��ȫʱ��pH | |
| Fe3�� | 2.6��10��39 | 1.9 | 3.2 |
| Cu2�� | 2.2��10��20 | 4.7 | 6.7 |
����һӦ������Һ��pH��Χ��__________�������ó����ܽ�ƽ����й����۽��ͼ���CuO�ܳ�ȥCuSO4��Һ��Fe3����ԭ��________________________________________________
________________________________________________________________________��
�ڲ������еľ������������_________________________________________________��
�� ( 1)Fe��S��������
1)Fe��S��������
(2)��3.2��pH��4.7������CuO��H����Ӧʹc(H��)��С��c(OH��)����ʹ��Һ��c(Fe3��)��c 3(OH��)��Ksp[Fe(OH)3]������Fe3�����ɳ�������ȥ
3(OH��)��Ksp[Fe(OH)3]������Fe3�����ɳ�������ȥ
������Ũ������ȴ�ᾧ������
���� (1)CuFeS2��FeΪ��2�ۣ�CuΪ��2�ۣ�SԪ��Ϊ��2�ۣ���Ӧ��CuԪ�ػ��ϼ۲��䣬FeԪ�ر�Ϊ��3�ۣ�SԪ�ر�Ϊ��6�ۣ��ʱ�������Ԫ����Fe��S��
(2)�ٲ���һ������ҺpH��Ŀ���ǽ�Fe3��ȫ��������������Cu2�����ܳ�������Ӧ����pH�ķ�ΧΪ3.2��pH��4.7���ڲ��������ǽ�����ͭ��Һ��Ϊ����ͭ���壬�ʲ��õķ���������Ũ������ȴ�ᾧ�����ˡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
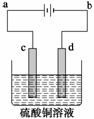
(1)[2012���¿α�ȫ������36(3)]��ͭ�ĵ�⾫����ͼ��ʾ���ڴ�ͭ�ĵ������У���ͭ��Ӧ��ͼ�е缫________(��ͼ�е���ĸ)���ڵ缫d�Ϸ����ĵ缫��ӦʽΪ__________������ͭ�л�����Au��Ag��Fe�������ڵ����еĴ�����ʽ��λ��Ϊ____________��
(2)(2012�����ϣ�16)����Ч�ļ���ȼ�ϵ�ز��ò�Ϊ�缫���ϣ����缫�Ϸֱ�ͨ��CH4��O2 �������ΪKOH��Һ��ij�о�С�齫��������ȼ�ϵ�ش�������Ϊ��Դ�����б����Ȼ�����Һ���ʵ�飬��ͼ��ʾ��
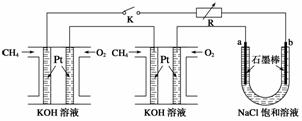
�ش��������⣺
�ټ���ȼ�ϵ�������������ĵ缫��Ӧ�ֱ�Ϊ________________________________��
________________________________________________________________________��
�ڱպ�K���غ�a��b�缫�Ͼ����������������b�缫�ϵõ�����__________������Ȼ�����Һ���ܷ�Ӧ����ʽΪ
________________________________________________________________________��
����ÿ����ؼ���ͨ����Ϊ1 L(��״��)���ҷ�Ӧ��ȫ����������ͨ�����صĵ���Ϊ__________(�����ڳ���F��9.65��104 C �� mol��1��ʽ����)������ܲ������������Ϊ________L(��״��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�˵���������
A��˳-2-��ϩ�ͷ�-2-��ϩ�ֱ���H2�ӳɣ�������ͬ�ļӳɲ���
B�������������顢��ϩ����Ȳ��ȫȼ�պ��������μ�С
C����������ȴ�����2��
D��(CH3)3CCl��(CH3)2CHCH2Cl�ֱ���NaOH���Ҵ���Һ���ȣ����ɲ�ͬ����ȥ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������C3H6�ϳ��л��߷���E��C6H14������ͼ����ش��������⣺
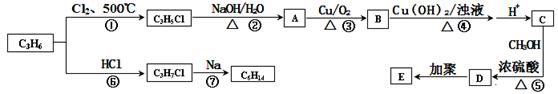
(1)C6H14�˴Ź�����ֻ�����ַ壬��C6H14�Ľṹ��ʽΪ��_________________,
д��E�Ľṹ��ʽ��___________________��
(2)д��B������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ��________________________��
(3)D��ͬ���칹��ܶ࣬��������������ͬ���칹����__________��
�ٺ�̼̼˫�� ����ˮ�� ���ܷ���������Ӧ
(4)��������ѧ֪ʶ����ͼ�������Ϣ�����Ҵ�Ϊ��Ҫԭ��ͨ���������ܺϳɻ�����(���Լ���ѡ����д����һ���͵�������ѧ��Ӧ�Ļ�ѧ����ʽ:
________________________________��________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��AgCl�ֱ����ʢ�У���5 mLˮ����6 mL 0.5 mol��L��1 NaCl��Һ����10 mL 0.2 mol��L��1 CaCl2��Һ����50 mL 0.1 mol��L��1������ձ��У����й���ʣ�࣬����Һ��c(Ag��)�Ӵ�С��˳��������ȷ���� (����)��
A���ܢۢڢ� B���ڢۢܢ� C���٢ܢۢ� D ���٢ۢڢ�
���٢ۢڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ��һ���ܴ����������(����)��
A��1.0 mol��L��1��KNO3��Һ��H����Fe2����Cl����SO
B�����ȳʺ�ɫ����Һ��NH ��Ba2����[Al(OH)4]����Cl��
��Ba2����[Al(OH)4]����Cl��
C��pH��12����Һ��K����Na����CH3COO����Br��
D��������Ӧ����������������Һ��Na����K����CO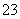 ��NO
��NO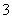
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������A��B��C��D��E�����ǵ������ӿ�����Na����NH4����Cu2����Ba2����Al3���� Ag����Fe3���������ӿ�����Cl����NO3����SO42����CO32������֪��
Ag����Fe3���������ӿ�����Cl����NO3����SO42����CO32������֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش��������⡣
(1)�������У�һ��û�е���������________��������������ͬ�������εĻ�ѧʽ��________��
(2)D�Ļ�ѧʽΪ________��D��Һ�Լ��Ե�ԭ����(�����ӷ���ʽ��ʾ)_______________________________________________________________
________________________________________________________________��
(3)A��C����Һ��Ӧ�����ӷ���ʽ��________��E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��________________________________________________________ _____��
_____��
(4)��Ҫ����B�������������ӣ���ȷ��ʵ�鷽����______________________
_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������������Ժ��ֱ�����ϣ�ֻҪ�ܵ����ʵ���һ������ȫȼ��ʱ�������������Ƕ�ֵ���ǣ� ��
A��CH2O��C2H4O2��C6H12O6
B��C6H6 ��C5H12 ��C7H6O2
C��CH2=CH2 ��C2H5OH��HOCH2CH2COOH
D��H2 ��CO CH3OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��aAn+��bB2-�������ӵĺ�����Ӳ�ṹ��ͬ����a����ֵΪ( )
A.b+n+2 B.b+n-2 C.b-n-2 D.b-n+2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com