

·ÖĪö ¹čŌåĶĮÖ÷ŅŖ³É·ÖŹĒSiO2ŗĶÓŠ»śÖŹ£¬²¢ŗ¬ÓŠÉŁĮæµÄAl2O3”¢Fe2O3”¢MgOµČŌÓÖŹ£¬½«¹čŌåĶĮŌŚŪįŪöÖŠģŃÉÕ£¬¹čŌåĶĮÖŠµÄÓŠ»śĪļ±»×ĘÉÕ£¬µĆµ½µÄ¹ĢĢåÖŠŗ¬ÓŠSiO2”¢Al2O3”¢Fe2O3”¢MgOµČĪļÖŹ£¬Č»ŗóĻņČÜŅŗÖŠ¼ÓČėĮņĖį£¬·¢ÉśµÄ·“Ó¦ÓŠAl2O3+3H2SO4=Al2£ØSO4£©3+3H2O”¢Fe2O3+3H2SO4=Fe2£ØSO4£©3+3H2O”¢MgO+H2SO4=MgSO4+H2O£¬SiO2²»ČÜÓŚĻ”ĮņĖį£¬Č»ŗó¹żĀĖµĆµ½¹ĢĢåĪŖSiO2£¬½«¹ĢĢåŗęøɵƵ½¾«ÖĘ¹čŌåĶĮ£»
ĻņĀĖŅŗÖŠ¼ÓČė¹żĮæNaOH£¬·¢ÉśµÄ·“Ó¦ĪŖAl3++4OH-=AlO2-+2H2O”¢Fe3++3OH-=Fe£ØOH£©3”ż”¢Mg2++2OH-=Mg£ØOH£©2”ż£¬Č»ŗó¹żĀĖ£¬ĀĖŅŗÖŠČÜÖŹĪŖNaAlO2”¢NaOH£¬ĻņĀĖŅŗÖŠĶØČė¹żĮ涞Ńõ»ÆĢ¼£¬·¢ÉśµÄ·“Ó¦ĪŖAl O2-+CO2+2H2O=Al£ØOH£©3”ż+HCO3-£¬¹żĮæµĆµ½ĒāŃõ»ÆĀĮ³Įµķ£¬ŌŁ½įŗĻĢāÄæ·ÖĪö½ā“š£®
½ā“š ½ā£ŗ¹čŌåĶĮÖ÷ŅŖ³É·ÖŹĒSiO2ŗĶÓŠ»śÖŹ£¬²¢ŗ¬ÓŠÉŁĮæµÄAl2O3”¢Fe2O3”¢MgOµČŌÓÖŹ£¬½«¹čŌåĶĮŌŚŪįŪöÖŠģŃÉÕ£¬¹čŌåĶĮÖŠµÄÓŠ»śĪļ±»×ĘÉÕ£¬µĆµ½µÄ¹ĢĢåÖŠŗ¬ÓŠSiO2”¢Al2O3”¢Fe2O3”¢MgOµČĪļÖŹ£¬Č»ŗóĻņČÜŅŗÖŠ¼ÓČėĮņĖį£¬·¢ÉśµÄ·“Ó¦ÓŠAl2O3+3H2SO4=Al2£ØSO4£©3+3H2O”¢Fe2O3+3H2SO4=Fe2£ØSO4£©3+3H2O”¢MgO+H2SO4=MgSO4+H2O£¬SiO2²»ČÜÓŚĻ”ĮņĖį£¬Č»ŗó¹żĀĖµĆµ½¹ĢĢåĪŖSiO2£¬½«¹ĢĢåŗęøɵƵ½¾«ÖĘ¹čŌåĶĮ£»
ĻņĀĖŅŗÖŠ¼ÓČė¹żĮæNaOH£¬·¢ÉśµÄ·“Ó¦ĪŖAl3++4OH-=AlO2-+2H2O”¢Fe3++3OH-=Fe£ØOH£©3”ż”¢Mg2++2OH-=Mg£ØOH£©2”ż£¬Č»ŗó¹żĀĖ£¬ĀĖŅŗÖŠČÜÖŹĪŖNaAlO2”¢NaOH£¬ĻņĀĖŅŗÖŠĶØČė¹żĮ涞Ńõ»ÆĢ¼£¬·¢ÉśµÄ·“Ó¦ĪŖAl O2-+CO2+2H2O=Al£ØOH£©3”ż+HCO3-£¬¹żĮæµĆµ½ĒāŃõ»ÆĀĮ³Įµķ£¬
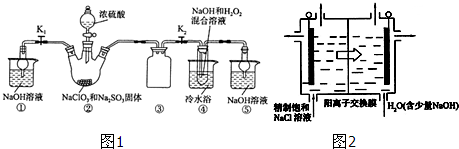
£Ø1£©ĶعżŅŌÉĻ·ÖĪöÖŖ£¬“Ö¹čŌåĶĮøßĪĀģŃÉÕµÄÄæµÄŹĒ³żČ„ÓŠ»śĪļ£¬ŹµŃéŹŅÖŠ½ųŠŠģŃÉÕµÄ×°ÖĆČēĶ¼2ĖłŹ¾£¬ŅĒĘ÷aµÄĆū³ĘŹĒŪįŪö£¬¹Ź“š°øĪŖ£ŗ³żČ„ÓŠ»śĪļ£»ŪįŪö£»
£Ø2£©·ÖĄėÄŃČÜŠŌ¹ĢĢåŗĶČÜŅŗ²ÉÓĆ¹żĀĖ·½·Ø£¬·“Ó¦¢ńŗóµĆ”°¹żĀĖ”±Ó¦øĆŹĒĘÕĶعżĀĖ£¬ŅņĪŖ¹čŌåĶĮæÅĮ£Š”£¬»į¶ĀČūĀĖÖ½£¬¹Ź“š°øĪŖ£ŗĘÕĶØ£»¹čŌåĶĮæÅĮ£Š”£¬»į¶ĀČūĀĖÖ½£»
£Ø3£©·“Ó¦¢ņŗó¹żĀĖ£¬ĖłµĆ¹ĢĢåµÄÖ÷ŅŖ³É·ÖŹĒFe£ØOH£©3”¢Mg£ØOH£©2£¬¹Ź“š°øĪŖ£ŗFe£ØOH£©3”¢Mg£ØOH£©2£»
£Ø4£©Ļ“µÓAl£ØOH£©3ŹĒĮ½ŠŌĒāŃõ»ÆĪļ£¬ÄÜČÜÓŚĻ”ŃĪĖįŗĶĒāŃõ»ÆÄĘČÜŅŗ£¬ĪŖŅÖÖĘĒāŃõ»ÆĀĮČܽā£¬Ó¦øĆÓĆĖ®Ļ“Č»ŗóÓĆŅŅ“¼Ļ“£¬ŅŅ“¼¾ßÓŠ»Ó·¢ŠŌ£¬µ¼ÖĀĒāŃõ»ÆĀĮŅ×øÉŌļ£¬¹ŹŃ”C£»
£Ø5£©Al£ØOH£©3“æ¶Č²ā¶Ø£ŗ×¼Č·³ĘČ”Ņ»¶ØÖŹĮæµÄѳʷ£¬ŌŚøßĪĀĻĀ³ä·Ö×ĘÉÕ£¬ĄäČ“ŗó³ĘÖŲ£¬
A£®¹ĢĢåÖŠŗ¬ÓŠNa2CO3ŌÓÖŹ£¬Ģ¼ĖįÄĘ²»·Ö½ā£¬»įµ¼ÖĀ¹ĢĢåÖŹĮæ¼õÉŁµÄĮæ½µµĶ£¬Ōņ²ā¶Ø½į¹ūĘ«µĶ£¬¹Ź“ķĪó£»
B£®¹ĢĢåÖŠŗ¬ÓŠNaHCO3ŌÓÖŹ£¬Ģ¼ĖįĒāÄĘŹÜČČŅ×·Ö½ā£¬ĻąĶ¬ÖŹĮæµÄĒāŃõ»ÆĀĮŗĶĢ¼ĖįĒāÄĘ£¬Ģ¼ĖįĒāÄĘ¼õÉŁµÄÖŹĮæÉĻ£¬ĖłŅŌµ¼ÖĀ²ā¶Ø½į¹ūĘ«øߣ¬¹ŹÕżČ·£»
C£®Al£ØOH£©3ŌŚ×ĘÉÕĒ°ŅŃ²æ·Ö·Ö½ā£¬»įµ¼ÖĀ¹ĢĢåŌŚ×ĘÉÕĒ°ŗóÖŹĮæ¼õÉŁ½µµĶ£¬Ōņ²ā¶Ø½į¹ūĘ«µĶ£¬¹Ź“ķĪó£»
D£®Al£ØOH£©3Ī“ĶźČ«øÉŌļ£¬»įµ¼ÖĀ¹ĢĢåŌŚ×ĘÉÕĒ°ŗóÖŹĮæ¼õÉŁŌö¼Ó£¬ĖłŅŌ³Įµķ½į¹ūĘ«øߣ¬¹ŹÕżČ·£»
¹ŹŃ”BD£»
£Ø6£©ÅŻÄĆš»šĘ÷ŹĒ½«“óĮæCO2ŗĶĒāŃõ»ÆĀĮµČŅŌÅŻÄµÄŠĪŹ½Åē³ö£¬ĒāŃõ»ÆĀĮ·Ö½āŹĒĪüČČ·“Ó¦£¬ĒŅ·Ö½āÉś³ÉµÄŃõ»ÆĀĮƻӊæÉČ¼ŠŌ£¬ĒŅ»įø²øĒæÉČ¼Īļ£¬Éś³ÉĖ®ÕōĘų»įÅÅæŖÖÜĪ§µÄæÕĘų£¬
¹Ź“š°øĪŖ£ŗ·Ö½āĪüČČ”¢Ńõ»ÆĀĮ»įø²øĒæÉČ¼Īļ”¢Éś³ÉĖ®ÕōĘų»įÅÅæŖÖÜĪ§µÄæÕĘų£®
µćĘĄ ±¾Ģāæ¼²éÖʱøŹµŃé·½°øÉč¼Ę£¬ĪŖøßĘµæ¼µć£¬²ąÖŲæ¼²éŹµŃ黳±¾²Ł×÷”¢ŹµŃéŌĄķ”¢ĪļÖŹŠŌÖŹ£¬Ć÷Č·ĪļÖŹŠŌÖŹ¼°ŹµŃé²Ł×÷¹ę·¶ŠŌŹĒ½ā±¾Ģā¹Ų¼ü£¬ŅדķµćŹĒĪó²ī·ÖĪö£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅ“¼ŗĶĖ® | B£® | ĻćÓĶŗĶŹ³ŃĪĖ® | ||
| C£® | »ĘŗÓĖ®ÖŠµÄÄąÉ³ÓėĖ® | D£® | Ź³ŃĪĖ®ÖŠ»ńµĆŹ³ŃĪ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
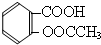 £©£®ŹµŃéŹŅŅŌĖ®ŃīĖį£ØĮŚōĒ»ł±½¼×Ėį£©Óė“×Ėįōū[£ØCH3CO£©2O]ĪŖÖ÷ŅŖŌĮĻŗĻ³ÉŅŅõ£Ė®ŃīĖį£¬ÖʱøµÄÖ÷ŅŖ·“Ó¦ČēĶ¼1£ŗ²Ł×÷Į÷³ĢČēĶ¼2£ŗ
£©£®ŹµŃéŹŅŅŌĖ®ŃīĖį£ØĮŚōĒ»ł±½¼×Ėį£©Óė“×Ėįōū[£ØCH3CO£©2O]ĪŖÖ÷ŅŖŌĮĻŗĻ³ÉŅŅõ£Ė®ŃīĖį£¬ÖʱøµÄÖ÷ŅŖ·“Ó¦ČēĶ¼1£ŗ²Ł×÷Į÷³ĢČēĶ¼2£ŗ £®
£® +3NaOH
+3NaOH CH3COONa+2H2O+
CH3COONa+2H2O+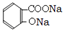 £®
£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

 CO32-+7H2O£®
CO32-+7H2O£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ³ĘČ”Ź³ŃĪ | B£® |  Ļ”ŹĶÅØĮņĖį | C£® |  ¼ģ²āĘųĆÜŠŌ | D£® |  µćČ¼¾Ę¾«µĘ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com