
��ͼ��ʾ���ס������ص缫���϶���������ʯī������ش��������⣺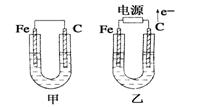
��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��__________________________________��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ______________________________��
�ڼ׳���ʯī�缫�ϵĵ缫��Ӧ����______(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ���KI������ֽ�����ҳ�ʯī�缫������������ֽ��������Ӧ�Ļ�ѧ����ʽΪ__________________________________��
�����ҳ�ת��0.02 mol e����ֹͣʵ�飬������Һ�����200 mL������Һ����Ⱥ��pH��_______��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ���ס������ص缫���϶���������ʯī������ش��������⣺
��ͼ��ʾ���ס������ص缫���϶���������ʯī������ش��������⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
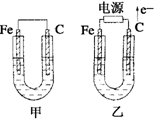 ��ͼ��ʾ���ס������ص缫���϶���������̼������ش��������⣺
��ͼ��ʾ���ס������ص缫���϶���������̼������ش��������⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(ÿ��3��,��21��)��ͼ��ʾ���ס������ص缫���϶���������̼������ش��������⣺
��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�______�����ҳ��е�______����
���ҳ��������ĵ缫��Ӧʽ��____________________��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ��___________________��
�ڼ׳���̼���ĵ缫��Ӧʽ��______________��
�۽�ʪ��ĵ��ۡ�KI��ֽ�����ҳ�̼��������������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�ֽ����ɵ�I2����������Ӧ��Cl2��I2���ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽ��_______________________________��
�����ҳ�ת��0.02mol e����ֹͣʵ�飬������Һ�����200mL����Ӧ����Һ��pH�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ���ס������ص缫���϶���������ʯī������ش��������⣺
��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��__________________________________��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ______________________________��
�ڼ׳���ʯī�缫�ϵĵ缫��Ӧ����______(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ���KI������ֽ�����ҳ�ʯī�缫������������ֽ��������Ӧ�Ļ�ѧ����ʽΪ__________________________________��
�����ҳ�ת��0.02 mol e����ֹͣʵ�飬������Һ�����200 mL������Һ����Ⱥ��pH��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�人�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��ͼ��ʾ���ס������ص缫���϶���������ʯī������ش��������⣺
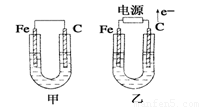
��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��__________________________________��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ______________________________��
�ڼ׳���ʯī�缫�ϵĵ缫��Ӧ����______(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ���KI������ֽ�����ҳ�ʯī�缫������������ֽ��������Ӧ�Ļ�ѧ����ʽΪ__________________________________��
�����ҳ�ת��0.02 mol e����ֹͣʵ�飬������Һ�����200 mL������Һ����Ⱥ��pH��_______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com