
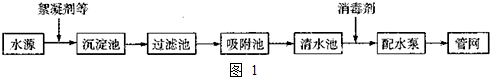
���� ��1�������ĺ���������Ϊ������Ư�ۡ��������ȵȣ�����̿���Գ�ȥ ˮ�е���ζ��K2FeO4֮��������ˮ������������Ϊ���������ǿ�����ԣ���ɱ��������ͬʱ�����������ԭ���������ӣ�������ˮ���γɽ�����ǿ�����ԣ��ܳ�ȥˮ�е����ʣ��ݴ˴��⣻
��2����ҪʹpH��ΧΪ6.5��8.5������ˮ������PH�ƻ���PH��ֽ�����ˮ��pH�Ƿ����Ҫ��
��Ca2+��Ũ��Ϊl��8��10-3mol•L-1=l��8��40��10-3g•L-1=72mg•L-1��450mg•L-1���ݴ��жϣ�
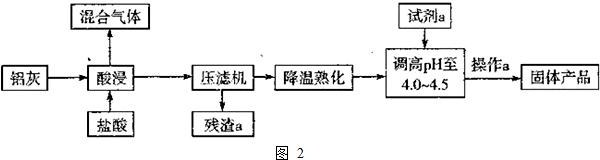
��3��������Ҫ��Al2O3��Al������SiO2�����ʣ��������ᣬ�����������������ᷴӦ���������費�������ᷴӦ�����Բ���Ϊ�������裬���˵��Ȼ�����Һ���ٵ���pH��Ŀ����Ҫ�õ��ۺ��Ȼ�����ͬʱ���������µ����ʣ����Կ�����Al2O3���پ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�Ӽ�����ɵò�Ʒ��
��� �⣺��1�������ĺ���������Ϊ������Ư�ۡ��������ȵȣ�����̿���Գ�ȥ ˮ�е���ζ��K2FeO4֮��������ˮ������������Ϊ���������ǿ�����ԣ���ɱ��������ͬʱ�����������ԭ���������ӣ�������ˮ���γɽ�����ǿ�����ԣ��ܳ�ȥˮ�е����ʣ�
�ʴ�Ϊ��������Ư�ۡ��������ȵȣ� ��ζ�������������ԭ���������ӣ�������ˮ���γɽ�����ǿ�����ԣ�
��2����ҪʹpH��ΧΪ6.5��8.5������ˮ������PH�ƻ���PH��ֽ�����ˮ��pH�Ƿ����Ҫ��
�ʴ�Ϊ��PH�ƻ���PH��ֽ��
��Ca2+��Ũ��Ϊl��8��10-3mol•L-1=l��8��40��10-3g•L-1=72mg•L-1��450mg•L-1�����Է��Ϲ���Ҫ��
�ʴ�Ϊ�����ϣ���ΪCa2+��Ũ��Ϊl��8��10-3mol•L-1=l��8��40��10-3g•L-1=72mg•L-1��450mg•L-1��
��3��������Ҫ��Al2O3��Al������SiO2�����ʣ��������ᣬ�����������������ᷴӦ���������費�������ᷴӦ�����Բ���Ϊ�������裬���˵��Ȼ�����Һ���ٵ���pH��Ŀ����Ҫ�õ��ۺ��Ȼ�����ͬʱ���������µ����ʣ����Կ�����Al2O3���پ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�Ӽ�����ɵò�Ʒ��
�����������Ҫ���貢���ȵ�95�����ң���������������ȡ�����ʣ�
�ʴ�Ϊ��������ȡ�����ʣ�
�ڸ�������ķ�����֪���Լ�a����������������ѡ b��
��ѹ�˻��Ĺ�����ʵ���еĹ�������������ͬ �ģ�����a������������Ũ������ȴ�ᾧ�����ˡ�ϴ�Ӽ����
�ʴ�Ϊ����ͬ������Ũ������ȴ�ᾧ�����ˣ�
���� ������ˮ������ˮ���ۺ��Ȼ���������Ʊ�Ϊ���壬���������仯�������ʡ����ӷ���ʽ���Թ������̵����⡢���ӵȣ��Ѷ��еȣ��ؼ����ڶԹ������̵����⣬��֪ʶ��Ǩ�����ã�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
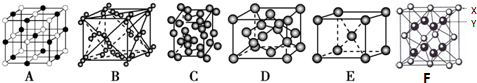
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 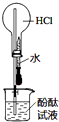 ��ͼʵ��ɹ۲쵽��ɫ��Ȫ | |
| B�� | 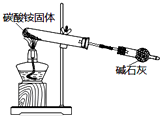 ��ͼʵ�����ȡ�������� | |
| C�� | 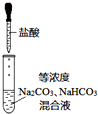 ��ͼʵ������εμ�ϡ����ʱ���Թ������������������� | |
| D�� | 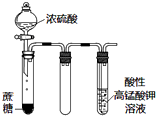 ��ͼʵ��������KMnO4��Һ���д������ݳ��֣�����Һ��ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
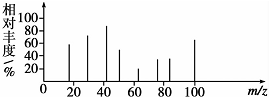
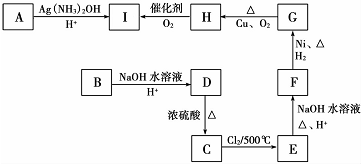
 ��HOCH2CH2CH2CH2COOH��
��HOCH2CH2CH2CH2COOH�� ��
��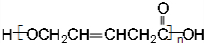 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4�� | B�� | 5�� | C�� | 6�� | D�� | 7�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
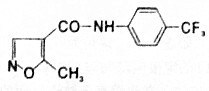
| A�� | ����ʽΪC12H10F3N2O2 | B�� | ���Է���ˮ�ⷴӦ��������Ӧ | ||
| C�� | �û�����������ˮ�������ھƾ� | D�� | �û���������̼ԭ�Ӳ����ܹ�ƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CaO | B�� | NaHCO3 | C�� | SiO2 | D�� | CaCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
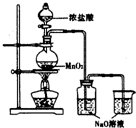 ijʵ��С������ͼװ���Ʊ���������Һ����̽�������ʣ���Ӧֹͣ��ȡϴ��ƿ����ɫ��Һ5mL�ֱ����������ʵ�飺
ijʵ��С������ͼװ���Ʊ���������Һ����̽�������ʣ���Ӧֹͣ��ȡϴ��ƿ����ɫ��Һ5mL�ֱ����������ʵ�飺| ���� | ���� |
| a������ҺpH���������еμ�2�η�̪ | pH=3����Һ��죬5min����ɫ |
| b����������μ������� | ��Һ��ɻ���ɫ |
| ���� | ���� |
| ȡ5mL pH=13NaOH��Һ�������еμ�2�η�̪ | ��Һ��죬30min����ɫ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com