
| ��� | ��Ҫʵ�鲽�輰ʵ������ |
| �� | �ں���B����Һ�У�����ϡH2S04������dz��ɫ���Ǻ���ɫ�д̼�����ζ�����壮 |
| �� | 20 ml��ˮ�еμ�F�ı�����Һ1��2ml������Һ��ʺ��ɫ |
| �� | ��ʵ��ڵõ��ĺ��ɫҺ��������������գ����յõ�����ɫ���� |
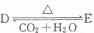 ������D��E?XH20�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ
������D��E?XH20�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ
| ||
| ||
| 1mol��5 |
| 2 |
| 3.42g |
| 18g/mol |
| 2.2g |
| 44g/mol |
| ||
| 2.12g |
| 106g/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
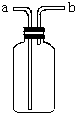 ��ͼ��ʾװ���ǻ�ѧʵ���г�������������������ϴ���⣬����������;��
��ͼ��ʾװ���ǻ�ѧʵ���г�������������������ϴ���⣬����������;���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
| A | ����CH3CH2Br��NaOH��Һ���Ƿ���ˮ�� | ��CH3CH2Br��NaOH��Һ���ȣ���ȴ��ȡ���ϲ�ˮ��Һ������AgNO3��Һ���۲��Ƿ��������ɫ���� |
| B | ����Fe��NO3��2�����Ƿ����������� | ��Fe��NO3��2��Ʒ����ϡH2SO4�μ�KSCN��Һ���۲���Һ�Ƿ��� |
| C | ��֤�����ԣ�Fe3+��I2 | ��KI��FeCl3��Һ���Թ��л�Ϻ������ã��ɹ۲���Һ��ɫ�仯 |
| D | ��֤Fe��OH��3���ܽ��С��Mg��OH��2 | ��FeCl3��Һ����Mg��OH��2����Һ�У����ɹ۲쵽�����ɰ�ɫ��Ϊ���ɫ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ػơ��غ� |
| B���ػơ��� |
| C���غ졢�� |
| D���غ졢�ػ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��NH4+����c��Cl-�� |
| B��c��H+��=c��OH-�� |
| C��c��NH4+��+c��NH3?H2O����c��Cl-�� |
| D��c��NH4+����c��Cl-��+c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���ͻ���� | |
| A | SO2ʹ���Ը��������Һ��ɫ | SO2���ֻ�ԭ�� |
| B | ŨHNO3�ڹ��������±�� | ŨHNO3���ȶ���������ɫ����������Ũ���� |
| C | ij��Һ�м���ŨNaOH��Һ���ȣ��ų�������ʹʪ��ĺ�ɫʯ����ֽ���� | ����Һ��һ������NH4+ |
| D | ��Ƭ����Ũ�����У������Ա仯 | ˵���������Ũ���������ѧ��Ӧ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Zn2++2OH-=Zn��OH��2�� ZnCO3+2NaOH=Zn��OH��2��+Na2CO3 |
| B��Ba2++SO42-=BaSO4�� Ba��OH��2+H2SO4=BaSO4��+2H2O |
| C��Ag++Cl-=AgCl�� AgNO3+NaCl=AgCl��+NaNO3 |
| D��Cu+2Ag+=Cu2++2Ag�� Cu+2AgCl=2Ag+CuCl2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com