
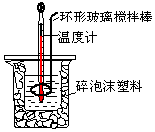
 ��50mL0.50mol/L������50mL 0.55mol/L NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ���ⶨǿ����ǿ�Ӧ�ķ�Ӧ�ȣ�
��50mL0.50mol/L������50mL 0.55mol/L NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ���ⶨǿ����ǿ�Ӧ�ķ�Ӧ�ȣ�| ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ� ��t2-t1��/�� | |||
| HCl | NaOH | ƽ��ֵ | |||
| 1 | 25.5 | 25.0 | 25.25 | 28.5 | 3.25 |
| 2 | 24.5 | 24.5 | 24.50 | 27.5 | 3.00 |
| 3 | 25.0 | 24.5 | 24.75 | 26.5 | 1.75 |
���� ��1����ȡ50mL�������NaOH ��Һʹ�ò�������Ϊ��Ͳ�ͽ�ͷ�ιܣ�
��2���к��Ȳⶨʵ��ɰܵĹؼ��Ǽ�����������ʧ��
��3���к��ȵIJⶨ�У���ȫ��Ӧ������Һ������¶�Ϊ��ֹ�¶ȣ�
��4�����ж����ݵ���Ч�ԣ�Ȼ������ƽ���¶Ȳ�ٸ���Q=m•c•��T���㷴Ӧ�ų���������
��5������NH3•H2O��������ʣ�������ʵ������Ƚ��з�����
��� �⣺��1���ڸ�ʵ���У���ȡ50mL�����NaOH��Һ����Ҫ�õ��IJ���������Ͳ�ͽ�ͷ�ιܣ�
�ʴ�Ϊ������ͽ�ͷ�ιܣ�
��2���к��Ȳⶨʵ���У�װ���д�С�ձ�֮����������ĭ���ϵ�Ŀ���Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��3������������Һ��������ȫ��Ӧ������Һ������¶�Ϊ��ֹ�¶ȣ�
�ʴ�Ϊ����ȫ��Ӧ������Һ����ߣ�
��4�������εõ��¶Ȳ����ϴ�Ӧ������������ƽ���¶Ȳ�Ϊ��$\frac{3.25��+3.1��}{2}$=3.125�棬��Һ������Ϊ100ml��1g/ml=100g�����Է�Ӧ�зų���������4.18J/��g•�棩��100g��3.125��=1306.25J=1.31kJ��
�ʴ�Ϊ��1.31��
��5��һˮ�ϰ�Ϊ����������Ϊ���ȹ��̣��ų�������ƫ�٣����к��ȡ�HΪ��ֵ����50mL 0.55mol/L�İ�ˮ��NH3•H2O������NaOH��Һ��������ʵ�飬��õ��к���ƫ��
�ʴ�Ϊ��һˮ�ϰ�Ϊ������ʣ���Ӧ�����е�����Ҫ�����������ʷų�������ƫ�٣�
���� ���⿼�����к��ȵIJⶨ����ȷ�к��ȵIJⶨ����Ϊ���ؼ���ע�������к��ȼ��㷽��������������ѧ���ķ�����������ѧʵ���������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��λʱ��������n mol AB��ͬʱ����n mol��B2 | |
| B�� | �����ڵ���ѹǿ����ʱ����仯 | |
| C�� | 2v��A2����=v��AB���� | |
| D�� | A2��B2��AB�ķ�Ӧ����֮��Ϊ1��1��2��״̬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O3?2H++CO32- | B�� | CH3COO-+H2O?CH3COOH+OH- | ||
| C�� | NaHCO3?Na++HCO3- | D�� | BaOH��2?Ba2++2OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ȡ����ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶ�Ҫ��ˮ�� | |
| B�� | �����������ʱ��Ӧʹ�¶ȼƵ�ˮ����������ƿ��ƿ�ڴ� | |
| C�� | ���з�Һ����ʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� | |
| D�� | ������������ʱ��Ӧ������������Һ���ɺ���ֹͣ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
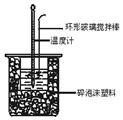 �к��Ȳⶨʵ���У���50mL0.50mol/L�����50mL0.55mol/LNaOH��Һ����ʵ�飬����˵������ȷ���ǣ�������
�к��Ȳⶨʵ���У���50mL0.50mol/L�����50mL0.55mol/LNaOH��Һ����ʵ�飬����˵������ȷ���ǣ�������| A�� | ����25mL 0.50mol/L�����25mL 0.55 mol/L NaOH��Һ���з�Ӧ��������к�����ֵ��ԭ����ͬ | |
| B�� | �����ʱ����Ͳ��NaOH��ҺӦ��������С�ձ��У������ò��������� | |
| C�� | װ���еĴ�С�ձ�֮����������ĭ���ϵ������DZ��¸��ȼ���������ʧ | |
| D�� | ʵ����Ӧ��¼������������¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶��С˳��W��Z��Y��X | |
| B�� | X��Y��W��������Ԫ���γ����������� | |
| C�� | W������������ˮ��������Ա�Z���� | |
| D�� | W�ֱ���X��Y�γɵĻ������л�ѧ��������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ٲ���NH4HS���� | B�� | ѹǿ�¶Ȳ����������Ar | ||
| C�� | �ݻ����¶�һ��������He | D�� | ���Ӳ���NH4HS���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com