
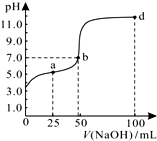
 �����£���100mL 0.01mol/L HA��Һ����μ���0.02mol/L NaOH��Һ�������Һ��pH�仯�������ͼ��ʾ�������ж���ȷ���ǣ�������
�����£���100mL 0.01mol/L HA��Һ����μ���0.02mol/L NaOH��Һ�������Һ��pH�仯�������ͼ��ʾ�������ж���ȷ���ǣ�������| A�� | ��ͼ����Ϣ��֪��HA����Ϊǿ�� | |
| B�� | a���Ӧ����Һ�У�2c��Na+��=c��A-��+c��HA�� | |
| C�� | b���Ӧ����Һ�У�c��Na+��=c��A-��+c��HA�� | |
| D�� | d������Ӧ����Һ������Ũ�ȵĴ�С��ϵΪ��c��Na+����c��A-����c��OH-����c��H+�� |
���� A������ͼ֪�������Һ������ʱV��NaOH�����С��50mL��˵�����ʱn��HA����n��NaOH������������Һ������ΪHR��NaA��NaAΪǿ�������Σ�
B��a����Һ�������ʱ��n��HA��=2n��NaOH���������Һ�д��������غ㣬���������غ��жϣ�
C��b����Һ�����ʱn��HA����n��NaOH�������������غ��жϣ�
D��d����Һ������Ϊ�����ʵ���Ũ�ȵ�NaOH��NaA����Һ�ʼ��ԣ���c��OH-����c��H+����������ӻ�ˮ������OH-������Һ��c��OH-����c��A-����
��� �⣺A������ͼ֪�������Һ������ʱV��NaOH�����С��50mL��˵�����ʱn��HA����n��NaOH������������Һ������ΪHR��NaA��NaAΪǿ�������Σ�����HAΪ���ᣬ��A����
B��a����Һ�������ʱ��n��HA��=2n��NaOH���������Һ�д��������غ㣬���������غ��2c��Na+��=c��A-��+c��HA������B��ȷ��
C��b����Һ�����ʱn��HA����n��NaOH�������������غ��c��Na+����c��A-��+c��HA������C����
D��d����Һ������Ϊ�����ʵ���Ũ�ȵ�NaOH��NaA����Һ�ʼ��ԣ���c��OH-����c��H+����������ӻ�ˮ������OH-������Һ��c��OH-����c��A-������Һ������Ũ�ȴ�С˳����c��Na+����c��OH-����c��A-����c��H+������D����
��ѡB��
���� ���⿼���������Һ�����жϣ�Ϊ��Ƶ���㣬���ؿ���ѧ��ͼ��������ж���������ȷ��Һ��ÿһ����Һ�����ʳɷּ��������ǽⱾ��ؼ���ע����Һ�д��ڵĵ���غ�������غ㣬�״�ѡ����D��ע����Һ��c��OH-����c��A-����Դ�С����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ����ƿ�ݻ� | �������� | ʵ����� |
| A | 480mL | ����ͭ��7.68g | ����500mLˮ |
| B | 480mL | ������12.0g | ���500mL��Һ |
| C | 500mL | ����ͭ��8.0g | ����500mLˮ |
| D | 500mL | ������12.5g | ���500mL��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ԣ�H3PO4��H3AsO4 | |
| B�� | �����ԣ�HClO4��HClO | |
| C�� | ��ԭ�ԣ�Na2S��Na2SO3 | |
| D�� | ���H+������OH-��CO${\;}_{3}^{2-}$��HCO${\;}_{3}^{-}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��4��������5��
��4��������5��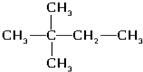 ��6����ˮ
��6����ˮ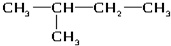 ��8��
��8�� ��9��${\;}_{17}^{35}$Cl
��9��${\;}_{17}^{35}$Cl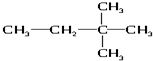
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
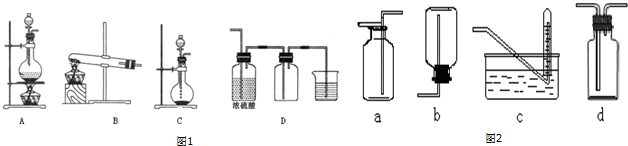
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ũ��ˮ�ķ�Ӧ | |
| B�� | �����������������±���������Ӧ | |
| C�� | ����������ȡ��ϩ | |
| D�� | �Ҵ���ͭ���������ȵ�������������ȩ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com