
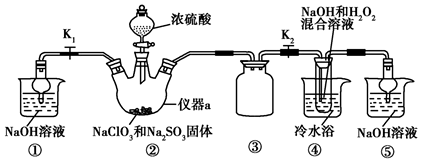
���� ��ʵ�������Ⱦ�����壬ֱ���ŷŻ���Ⱦ����������Ҫβ��������װ�âپ����ü����շ�Ӧ������ClO2��β�����ر�K1��װ�â��з�����Ӧ��2NaClO3+Na2SO3+H2SO4=2ClO2+2Na2SO4+H2O������ClO2���壬ClO2���徭װ�â۽���װ�âܣ�������Ӧ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2����NaClO2��Һ������ѹ��55�������ᾧ�����ȹ��ˣ� ��38�桫60�����ˮϴ�ӣ�����60�����þ���NaClO2•3H2O���ݴ˷������
��� �⣺��1������aΪ������ƿ��װ�âٺ�װ�âݶ������ն����ClO2���壬��ֹ��Ⱦ������װ�â۵������Ƿ�ֹ������
�ʴ�Ϊ��������ƿ�����ն����ClO2���壬��ֹ��Ⱦ��������ֹ������
��2��װ�â��в���ClO2����ѧ����ʽΪ��2NaClO3+Na2SO3+H2SO4��Ũ���T2ClO2��+2Na2SO4+H2O��װ�â���ΪClO2�������������������ķ�Ӧ����ѧ����ʽΪ��2NaOH+2ClO2+H2O2�T2NaClO2+2H2O+O2��
�ʴ�Ϊ��2NaClO3+Na2SO3+H2SO4��Ũ���T2ClO2��+2Na2SO4+H2O��2NaOH+2ClO2+H2O2�T2NaClO2+2H2O+O2��
��3����֪��NaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2•3H2O������38��ʱ�����ľ�����NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl���ʴ�װ�âܷ�Ӧ�����Һ��þ���NaClO2�IJ�������Ϊ����ѹ��55�������ᾧ�����ȹ��ˣ� ��38�桫60�����ˮϴ�ӣ�����60�����õ���Ʒ�������ȥ���е���ˮԡ�����ܵ��²�Ʒ�л��е�������NaClO3��NaCl��
�ʴ�Ϊ����38�桫60�����ˮϴ�ӣ�NaClO3��NaCl��
��4����������NaClO2�����Ƿ�������Na2SO4��ȡ����������������ˮ���μӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4��
�ʴ�Ϊ���μӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4��
��5��NaClO2�������ữ��KI��Һ����ӦΪ��ClO2-+4I-+4H+��2H2O+2I2+Cl-������Ʒ��NaClO2�����ʵ���x����
NaClO2��2I2��4S2O32-��
1mol 4mol
x 20��10-3��0.2mol
��ã�x=1��10-3mol��
10mL��Ʒ��m��NaClO2��=0.001mol��90.5g/mol=0.0905g��ԭ��Ʒ��NaClO2����������Ϊ��$\frac{0.0905g��1000}{10g}��100%$=90.5%��
�ʴ�Ϊ��90.5%��
���� ���⿼�����������Ʊ�ʵ��Ļ����������������Ƶ����ʼ��к͵ζ���֪ʶ������ԭ���ǽ���Ĺؼ���ͬʱ����ѧ���������⡢����������������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | С�Թ� | B�� | ����ƿ | C�� | ��Ͳ | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
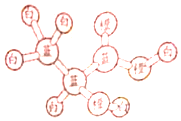 ij�л�����ӵ����ģ����ͼ��ʾ��ͼ�С�����������ѧ������ͬ��ɫ�ġ�������ͬԪ�ص�ԭ�ӣ�����˵��������ǣ�������
ij�л�����ӵ����ģ����ͼ��ʾ��ͼ�С�����������ѧ������ͬ��ɫ�ġ�������ͬԪ�ص�ԭ�ӣ�����˵��������ǣ�������| A�� | 1mol���л������2molNa��Ӧ������1mol���� | |
| B�� | ���л�����Է����Ӿ۷�Ӧ | |
| C�� | ���л�����Է���ȡ����Ӧ��������Ӧ��������Ӧ | |
| D�� | ���л���������ɷ���ʽΪC6H8O4���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �þƾ���������������������ۻ��������䣬˵��Al2O3��Al | |
| B�� | ��¯ˮ��CaSO4����Na2CO3��Һ���ݣ��������ܽ�ȥ����˵��Ksp��CaCO3��CaSO4 | |
| C�� | ��Na2SiO3��Һ��ͨ������CO2���壬�а�ɫ�������ɣ�˵�����ԣ�H2CO3��H2SiO3 | |
| D�� | ������ͨ��������Һʱ������һ�������ġ�ͨ·����˵����ɢ������ֱ����1-100nm֮�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
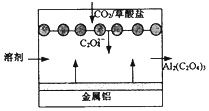
| A�� | ��װ���ǽ�����ת��Ϊ��ѧ�� | |
| B�� | �����ĵ缫��ӦΪ��C2O42--2e-=2CO2 | |
| C�� | ÿ�õ�1 mol����������·��ת��3 mol���� | |
| D�� | ���øü����ɲ������е�CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2NaBr+Cl2�TBr2+2NaCl | |
| B�� | AlCl3+3NaAlO2+6H2O�T4Al��OH��3��+3NaCl | |
| C�� | 2H2S+SO2�T3S��+2H2O | |
| D�� | Cl2+H2O�THCl+HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Mg2+��Na+��OH-��Cl- | B�� | Ag+��K+��Cl-��NO3- | ||
| C�� | Cu2+��NO3-��SO42-��Cl- | D�� | Na+��K+��CO32-��NO3- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com