
Ϊ�ⶨ ij�л�������A�Ľṹ����������ʵ�飺
ij�л�������A�Ľṹ����������ʵ�飺
(һ)����ʽ��ȷ����
(1)���л���A�����������г��ȼ�գ�ʵ���ã�����5.4 g H2O��8.8 g CO2����������6.72 L(��״����)����������и�Ԫ�ص�ԭ�Ӹ�������________��
(2)�������Dzⶨ���л����������Է����������õ� ��ͼ1��ʾ����ͼ��������Է�������Ϊ________�������ʵķ���ʽ��________��
��ͼ1��ʾ����ͼ��������Է�������Ϊ________�������ʵķ���ʽ��________��
(3)���ݼۼ����ۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ______________________��
(��)�ṹʽ��ȷ����
(4)�˴Ź��������ܶ��л�������в�ͬλ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ(�ź�)�����ݷ�ֵ(�ź�)����ȷ����������ԭ�ӵ��������Ŀ�����磺���ȼ���(Cl��CH2��O��CH3)��������ԭ����ͼ2�����ⶨ���л���A�ĺ˴Ź�������ʾ��ͼ��ͼ3����A�Ľṹ��ʽΪ________��
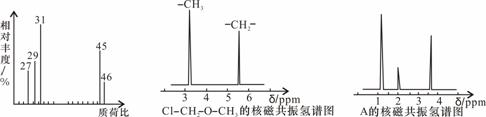 |
ͼ1 ͼ2  ͼ3
ͼ3
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±�Ϊ�������������������Լ���ȥ��Щ����ѡ�õ��Լ��������������ȷ����
| ���� | ���� | �Լ��� | |
| A | NaHCO3��Һ | Na2CO3 | ͨ�������CO2 |
| B | FeCl3��Һ | CuCl2 | Fe |
| C | Fe2O3 | Al2O3 | ���� |
| D | ��ˮ | Br2 | �Ҵ�����ȡ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������жϣ����и�Ԫ��һ����������Ԫ�ص��ǣ� ��
A��XԪ�����γ�+7�۵ĺ������ἰ����
B��YԪ��ԭ�����������2������
C��ZԪ�ص���������ͬһ����ϡ������Ԫ��ԭ�ӵĵ��Ӳ�ṹ��ͬ
D��RԪ��λ�����ڱ��е�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ �������������͡��������ܣ�
�ϳɶ�����ԭ���� �� ��
��CH2=CH��CH=CH2 ��CH3��C��C��CH3 ��CH2=CH��CN
��CH3-CH=CH-CN ��CH3��CH=CH2 ��CH3��CH=CH��CH3
A���٢ۢ� B���٢� C���٢ܢ� D���ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�۲����нṹ��ʽ���ش��������⣺
 |
������Ľṹ��ʽΪ 
��1��a��������__________��
��2��c��������________________��
��
��1�����������__________________________��
��2�����л���Ϊϩ���ӳɵIJ����ԭ��ϩ���Ľṹ������_______�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧʽΪC8H10�ķ������������ϵ�һ����ȡ����ֻ��һ�֣��÷�������������
A���ұ� B����-���ױ� C����-���ױ� D����-���ױ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����
A����������Ҫ��Դ�ڷ���ú���ͺ�ʯ�͵Ĵ�����
B������ͬϵ����ɱ����Ը��������Һ����
C���������������Ƿ�����
D���÷���ɸ����������������ұ����ɴ���Ƚ��ͶԻ�������Ⱦ�������Ч��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Na��Mg��Al���й����ʵ�������ȷ����(����)
A�����ԣ�NaOH<Mg(OH)2<Al(OH)3 B����һ�����ܣ�Na<Mg<Al
C���縺�ԣ�Na>Mg>Al D����ԭ�ԣ�Na>Mg>Al
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
W��X��Y��Z�Ƕ�����Ԫ�أ��䲿���������ұ�������˵����ȷ����
A����̬�⻯������ȶ��ԣ�X<W
B������������Ӧˮ��������ԣ�Y��X
C�����Ӱ뾶��Z��W
D��Y���������к��зǼ��Թ��ۼ�
| W | �����ǵ���ɫ���� |
| X | �ڵؿ��еĺ����ӵڶ�λ |
| Y | ԭ�������������ǵ���������2/3 |
| Z | ��������ԭ�Ӱ뾶��С�Ľ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com