���
�⣺A���������зų�46.3kJ�����������ɰ��������ʵ���Ϊ��2mol��
=1mol����
?X
2��g��+3Y
2��g��
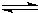
2XY
3��g��
��ʼ��1mol 3mol 0
ת����0.5mol 1.5mol 1mol
ƽ�⣺0.5mol 1.5mol 1mol
���������㣬��֪ƽ��ʱ��������X
2��Y
2��XY
3�����ʵ����ֱ�Ϊ0.5mol��1.5mol��1mol�����а���ѧ������ת������ߣ�n��X
2��=0.4mol+1.2mol��
=1mol��n��Y
2��=1.2mol+1.2mol��
=3mol����ƽ������ȫ��Чƽ�⣬��ƽ��ʱ��������X
2��Y
2��XY
3�����ʵ���Ҳ�ֱ�Ϊ0.5mol��1.5mol��1mol����ƽ��ʱ����������X
2���������Ϊ
����A��ȷ��
B�����º����£����а���ѧ������ת������ߣ�n��X
2��=0.4mol+1.2mol��
=1mol��n��Y
2��=1.2mol+1.2mol��
=3mol����ƽ������ȫ��Чƽ�⣬��ƽ��ʱ������������XY
3�����ʵ���Ũ����ȣ���B��ȷ��
C���������зų�46.3kJ�����������ɰ��������ʵ���Ϊ��2mol��
=1mol����
?X
2��g��+3Y
2��g��
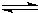
2XY
3��g��
��ʼ��1mol 3mol 0
ת����0.5mol 1.5mol 1mol
ƽ�⣺0.5mol 1.5mol 1mol
���������㣬��֪ƽ��ʱ��������X
2��Y
2��XY
3�����ʵ����ֱ�Ϊ0.5mol��1.5mol��1mol�������Ϊ��ȫ��Чƽ�⣬���ԣ�ƽ��ʱ��������X
2��Y
2��XY
3�����ʵ���Ҳ�ֱ�Ϊ0.5mol��1.5mol��1mol�������١����з�Ӧ��ƽ�ⳣ����ȣ�K=
=
����C����
D�����������Ϊ0.3L����Сѹǿƽ�����淴Ӧ�����ƶ�����Ӧ���ת���ʽ��ͣ���ƽ��ʱ�ų�������С��46.3kJ����D��ȷ��
��ѡC��

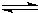 2XY3��g��
2XY3��g�� 2XY3��g��
2XY3��g��


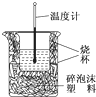 ��1���ڲⶨ�к��ȵ�ʵ���У�ʹ��������Ʒ����Ϊ�˼�Сʵ��������
��1���ڲⶨ�к��ȵ�ʵ���У�ʹ��������Ʒ����Ϊ�˼�Сʵ�������� ��
��