
����Ŀ����ͭ����������ʹ�õĺϽ�֮һ,��Ҫ��п��ͭ��ɡ��ش��������⣺
(1) ��̬пԭ�ӵĺ���۵����Ų�ʽΪ_______________,�������ڱ�__________��Ԫ�ء�����ռ������ܲ�ķ�����_______________ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ______________
(2)��һ������I1(Zn)________I1(Cu)(��������������С����)
(3)����ɫ ![]() ����ͭ��Һ�м����Թ����İ�ˮ,��Һ��Ϊ����ɫ[Cu(NH3)4] 2+��
����ͭ��Һ�м����Թ����İ�ˮ,��Һ��Ϊ����ɫ[Cu(NH3)4] 2+��
������������SO42-��Ϊ�ȵ��������__________(�����)��
A.H2SO4 B.CO32- C.PO43- D.CCl4
��H2O��������ԭ�ӵ��ӻ�����Ϊ______��NH3���ӵĿռ乹��Ϊ________��
�����еļ��ǣ�H2O_______ NH3(��������������С����)��
��ͨ������ʵ�������֪����Cu2+�����λ����H2O_________NH3 (��������������С����)��
������Ӧ��ǰ���İ����飨BH3��NH3�������黥Ϊ�ȵ����塣д��BH3��NH3�Ľṹʽ���ṹ��������λ������![]() ����ʾ��_______________________
����ʾ��_______________________
(4)����Cu�����е�ԭ�Ӷѻ���ʽ��ͼ��ʾ,���ֶѻ���ʽ��Ϊ_____________��

(5)��Cu������ܶ�Ϊ��g/cm3,![]() ��ʾ�����ӵ�������ֵ,��ʽ��ʾCu���������������Cuԭ��֮��ľ���________nm(���ػ���)
��ʾ�����ӵ�������ֵ,��ʽ��ʾCu���������������Cuԭ��֮��ľ���________nm(���ػ���)
���𰸡�![]() ds N ���� ���� CD sp3 ������ С�� С��
ds N ���� ���� CD sp3 ������ С�� С�� 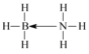 �����������ܶѻ�
�����������ܶѻ� 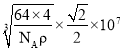
��������
��1����̬пԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s2�������۵����Ų�ʽΪ![]() ���ݴ���֪���������ڱ�����һ��Ԫ�أ����Ӳ��������⣬��������
���ݴ���֪���������ڱ�����һ��Ԫ�أ����Ӳ��������⣬��������
��2��п�ļ۵�����3d104s2��ͭ����3d104s1����ͭ������������ʧȥһ�����ӣ���һ�����п�Ĵ�
��3���ٵȵ�����ָ�������������ϵķ��ӻ����ӣ����ǵ�ԭ����Ŀ��ͬ���۵�����ĿҲ��ͬ���ݴ˽��з�����
�ڸ���ˮ���ӡ��������к��еĦҼ��µ��Ӷ������������ԭ���ӻ����ɣ��¶��Ӷ���Խ�࣬����ԽС���ݴ˷������ַ��ӵļ��Ǵ�С��
����[Cu��NH3��4]2+��Cu2+�ṩ�չ����NH3��Nԭ���ṩ�µ��Ӷԣ�����֮���γ���λ����
�ܰ����飨BH3��NH3�������黥Ϊ�ȵ����壬�����ӽṹ���ƣ���������ṹ�ķ��Ӿ��壬�����������N��Bԭ�Ӿ����������ӣ�Nԭ���й¶Ե��ӣ�Bԭ���пչ�����ݴ�д����ṹʽ��
��4��ͭ������ԭ�ӵĶѻ�ģ���������������ܶѻ���
��5�����ݾ�̯������������к���Cuԭ����Ŀ������������������Ӷ�����������ܶȣ�������= ![]()
![]() ,V=a3��10-21���м��㣻
,V=a3��10-21���м��㣻
��1����̬пԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s2�������۵����Ų�ʽΪ![]() ���������ڱ�ds��Ԫ�أ����Ӳ���ŷֱ�ΪK,L,M,N,O,P,Q����пԭ��������ߵ��������N�㣬ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ���Ρ�
���������ڱ�ds��Ԫ�أ����Ӳ���ŷֱ�ΪK,L,M,N,O,P,Q����пԭ��������ߵ��������N�㣬ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ���Ρ�
�ʴ�Ϊ��![]() ��ds ��N�����Σ�
��ds ��N������
��2��п�ļ۵�����3d104s2��ͭ����3d104s1����ͭ������������ʧȥһ�����ӣ���һ������I1(Zn)��I1(Cu)��
�ʴ�Ϊ�����ڣ�
��3���ٵȵ�����ָ�������������ϵķ��ӻ����ӣ����ǵ�ԭ����Ŀ��ͬ���۵�����ĿҲ��ͬ�����������Ƶĵ��ӽṹ�����Ƶļ��ι��ͣ�������ʱ��������Ҳ����������֮���� SO42-����5��ԭ�ӣ�32���۵��ӣ�����CD��ѡ����PO43- �ļ۵���Ϊ5+24+3=32��CCl4�ļ۵���Ϊ4��4��7=32��
��H2O��ֻ���е���Ϊ�Ҽ���������Oԭ�Ӻ���2���Ҽ����ӶԺ�2�Թµ��Ӷ����ӻ�����Ϊsp3�����ӹ���ΪV�ͣ�NH3���ӵĿռ乹��������������ԭ��Nԭ�Ӳ�ȡSP3�������ӻ���NH3��H2O����������ԭ�Ӷ���SP3�ӻ����������Ϊ�������壬������ȣ�NH3�д���1�Թµ��Ӷԣ�H2O�д���2�Թµ��Ӷԣ��¶��Ӷ���Խ�࣬����ԽС�����Լ���NH3>H2O��
����[![]() ����ͭ��Һ�м����Թ����İ�ˮ,��Һ��Ϊ����ɫ[Cu(NH3)4] ��������λ����ǿ��
����ͭ��Һ�м����Թ����İ�ˮ,��Һ��Ϊ����ɫ[Cu(NH3)4] ��������λ����ǿ��
�ܰ����飨BH3��NH3�������黥Ϊ�ȵ����壬�����ӽṹ���ƣ���������ṹ�ķ��Ӿ��壬�����������N��Bԭ�Ӿ����������ӣ�Nԭ���й¶Ե��ӣ�Bԭ���пչ�����ʽṹʽΪ ��
��
�ʴ�Ϊ��CD��sp3�� �����Σ�С�ڣ�С�ڣ� ��
��
��4����ͭ������ͭԭ�ӵĶѻ���ʽͼ��֪��ͭ������ԭ�ӵĶѻ�ģ���������������ܶѻ���
span>�ʴ�Ϊ�����������ܶѻ���
��5���辧���ⳤΪa nm��������Cuԭ����ĿΪ4����������Ϊ![]() g���ɼ���
g���ɼ���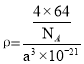 ����
����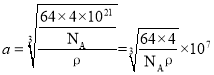 ���������ͭԭ��֮��ľ���Ϊ��Խ��߳��ȵ�һ�룬���������ͭԭ��֮��ľ���Ϊ
���������ͭԭ��֮��ľ���Ϊ��Խ��߳��ȵ�һ�룬���������ͭԭ��֮��ľ���Ϊ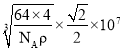 ��
��
�ʴ�Ϊ��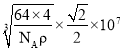 ��
��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������21.6 g��CO��CO2��ɵĻ�����壬�ڱ�״���������Ϊ13.44 L���ش��������⣺
(1)�û�������ƽ��Ħ������Ϊ________��
(2)���������̼ԭ�ӵ�����Ϊ________��
(3)�������������ͨ����ͼ��ʾװ�ã�����ռ���������(ʵ���ڱ�״���²ⶨ)��
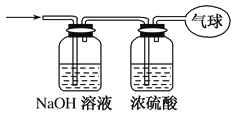
���������ռ����������Ħ������Ϊ________��
���������ռ����������У���������Ϊ________(��NA��ʾ�����ӵ�������ֵ)��
����������Ϊ________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����ѧ��ѧ�г����ڻ�����ķ�����ᴿװ�ã������װ�ûش����⣺
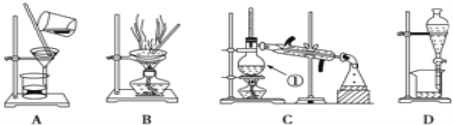
��ͼ����ѧ��ѧ�г����ڻ�����ķ�����ᴿװ�ã������װ�ûش����⣺
��1��װ��B�б�����������������_____��װ��C�Тٵ�������_________��
��2��ij�����ƹ����л����������������ʣ������һʵ�鷽�����ȳ�ȥ���ʣ��������������Һ��
ʵ�鷽�����Ƚ�������������ˮ�����Һ��ѡ����ʵ��Լ��Ͳ�����ɱ����и���ʵ�顣
ѡ���Լ� | �� | Na2CO3��Һ | �� |
ʵ����� | �� | �� | ���� |
��������Լ��ٿ�����_____���ѧʽ����֤����Һ��SO42-�Ѿ������IJ�����__________������Na2CO3��Һ��Ŀ����__________����������Լ�����____________ (�ѧʽ)��
��3���������Ǹ�Ч�Ŀ�ű��ҩ��Ϊ��ɫ��״���壬�����ڱ�ͪ���ȷºͱ��У��ڼ״����Ҵ������ѡ�ʯ�����п��ܽ⣬��ˮ�м������ܣ��۵�Ϊ156-157�棬���ȶ��Բ��֪�����ѷе�Ϊ35�档��ȡ�����ص���Ҫ����Ϊ��
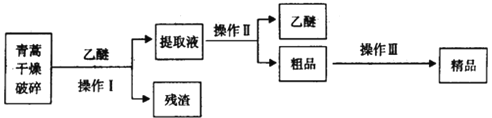
��Ҫ��ʵ����ģ���������գ�����Iѡ���ʵ�������_____��������ѡ���ʵ�������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ4NH3 (g) +5O2(g)![]() 4NO(g) +6H2O(g)�� ��H=-a kJmol-1����5L�ܱ�����Ͷ��1molNH3��1mol��O2��2���Ӻ�NO�����ʵ���������0.4mol������˵����ȷ����
4NO(g) +6H2O(g)�� ��H=-a kJmol-1����5L�ܱ�����Ͷ��1molNH3��1mol��O2��2���Ӻ�NO�����ʵ���������0.4mol������˵����ȷ����
A. 2���ӷ�Ӧ�ų�����.��ֵС��0.1akJ
B. ��������ʾ2���ӵķ�Ӧ���ʣ�v(O2)=0.05mol ��L-1 ��min-1
C. 2������NH3��ת������50��
D. 2����ĩ c(H2O)=0.6mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ������Ȼ������Ҫ��������������Ҫ�ɷ�ΪAl2O3��������Fe2O3��FeO��SiO2���С���ҵ�����������Ʊ�����ij�ֻ�����Ĺ����������¡�
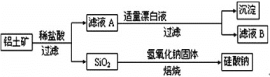
(1)����ҺA�м���Ư��Һ��������ҺB�����ԡ�
����ҺA�м���Ư��Һ��Ŀ���ǣ�________________________�������ӷ���ʽ��ʾ����
������ҺB�е���Ԫ���Գ�����ʽ��������ѡ�õ�����Լ�Ϊ������ţ�___________��
A������������Һ B��������Һ C����ˮ D��������̼
������Ӧ�����ӷ���ʽΪ____________
(2)������ҺB���Ƿ���Fe2+��ѡ����Լ�������Ϊ��_______________________________________
(3)������������������������������Һ�����Ӧ�����ӷ���ʽΪ________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д�����л�ѧ����ʽ�����ӷ���ʽ��
��1������ˮ��Ӧ�����ӷ���ʽ��___��
��2����������ͭ��Һ��Ӧ�����ӷ���ʽ��___��
��3�����������������̼��Ӧ�Ļ�ѧ����ʽ��___��
��4�������������������Ӧ�Ļ�ѧ����ʽ��___��
��5��þ�ڵ����е�ȼþ���Ļ�ѧ����ʽ��___��
��6��þ�ڶ�����̼�е�ȼþ���Ļ�ѧ����ʽ��___��
��7����������������Һ�ķ�Ӧ�����ӷ���ʽ��___��
��8��������������������Һ��Ӧ�����ӷ���ʽ��___��
��9����������������������Һ��Ӧ�����ӷ���ʽ��___��
��10������������������Ӧ�Ļ�ѧ����ʽ��___��
��11��ͭ���Ȼ�����Һ��Ӧ�����ӷ���ʽ��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ũ��������250mL0.1mol/L��������Һ��
��1������250mL0.1mol/L������Һ��ҪŨ���ᣨ�ܶ�Ϊ1.2g/mL����������Ϊ36.5%�������Ϊ__��������С�����һλ��
��2������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�___��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳ��ȡ�����Ũ���ᣬ�ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��250mL������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��3���������ʵ���Ũ�ȵ���Һ�����Ũ��ƫ�ߵIJ�����___������ѡ��
A���ܽ�����Һδ�������¾�ת������ƿ�У�
B��ϴ���ձ��Ͳ�������Һδת������ƿ�У�
C������ʱ�۾����ӿ̶��ߣ�
D������ʱ�۾����ӿ̶��ߣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����![]() ��ʾ�����ӵ�������ֵ������������ȷ���ǣ� ��
��ʾ�����ӵ�������ֵ������������ȷ���ǣ� ��
A.����NA����ԭ�ӵĵ����ڱ�״���µ����ԼΪ22.4L
B.��״���£�11.2L![]() ���еķ�����Ϊ0.5NA
���еķ�����Ϊ0.5NA
C.�ڳ��³�ѹ�£�11.2 L![]() ���еķ�����Ϊ0.5NA
���еķ�����Ϊ0.5NA
D.![]() ��
��![]() �Ļ���������ԭ����Ϊ0.2NA
�Ļ���������ԭ����Ϊ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾA��D�е�װ�ý��л����ķ�����ᴿ����Ҫ��ش��������⣺
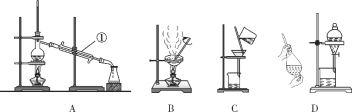
(1)�����ٵ�����______________��
(2)��ȥCa(OH)2��Һ��������CaCO3����Ӧѡ��װ��________(����ĸ���ţ���ͬ)��
(3)���뻥�ܵ�����(�е�118 ��)����������(�е�77.1 ��)�Ļ����Ӧѡ��װ��________��
(4)��CCl4��ȡ��ˮ�еĵⵥ��Ӧѡ��װ��________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com