
·ÖĪö ĪŽÉ«³ĪĒåČÜŅŗ£¬Ņ»¶Ø²»“ęŌŚ£ŗCu2+”¢Fe3+£¬
£Ø1£©Č”²æ·ÖČÜŅŗ£¬ĻņĘäÖŠÖšµĪµĪČėBaCl2ČÜŅŗÖĮ¹żĮ棬ӊ°×É«³ĮµķÉś³É£¬“ęŌŚSO42-”¢CO32-ÖŠµÄÖĮÉŁŅ»ÖÖ£»
£Ø2£©Č”£Ø1£©·“Ó¦ŗó¹żĀĖĖłµĆ³Įµķ£¬ŌŚ³ĮµķÖŠ¼ÓČėĻ”ŃĪĖįŗ󣬳Įµķ²æ·ÖČܽā£¬Ö¤Ć÷“ęŌŚSO42-”¢CO32-£¬Ņ»¶Ø²»“ęŌŚ£ŗCa2+£»
£Ø3£©Č”£Ø1£©·“Ó¦ŗóµÄĀĖŅŗ£¬¼ÓAgNO3ČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś£¬¼ĢŠų¼ÓHNO3£¬³Įµķ²»Čܽā£¬²»ÄÜČ·¶ØŹĒ·ń“ęŌŚĀČĄė×Ó£¬øł¾ŻČÜŅŗµēÖŠŠŌŌĄķ£¬Ņ»¶Ø“ęŌŚK+£®
½ā“š ½ā£ŗĪŽÉ«³ĪĒåČÜŅŗ£¬Ņ»¶Ø²»“ęŌŚ£ŗCu2+”¢Fe3+£¬
£Ø1£©Č”²æ·ÖČÜŅŗ£¬ĻņĘäÖŠÖšµĪµĪČėBaCl2ČÜŅŗÖĮ¹żĮ棬ӊ°×É«³ĮµķÉś³É£¬“ęŌŚSO42-”¢CO32-ÖŠµÄÖĮÉŁŅ»ÖÖ£»
£Ø2£©Č”£Ø1£©·“Ó¦ŗó¹żĀĖĖłµĆ³Įµķ£¬ŌŚ³ĮµķÖŠ¼ÓČėĻ”ŃĪĖįŗ󣬳Įµķ²æ·ÖČܽā£¬Ö¤Ć÷“ęŌŚSO42-”¢CO32-£¬Ņ»¶Ø²»“ęŌŚ£ŗCa2+£»
£Ø3£©Č”£Ø1£©·“Ó¦ŗóµÄĀĖŅŗ£¬¼ÓAgNO3ČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś£¬¼ĢŠų¼ÓHNO3£¬³Įµķ²»Čܽā£¬²»ÄÜČ·¶ØŹĒ·ń“ęŌŚĀČĄė×Ó£®
¢ŁøĆČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄĄė×ÓÓŠ K+”¢SO42-”¢CO32-£¬¹Ź“š°øĪŖ£ŗK+”¢SO42-”¢CO32-£»
¢ŚæĻ¶Ø²»“ęŌŚµÄĄė×ÓÓŠ Ca2+”¢Cu2+”¢Fe3+£¬¹Ź“š°øĪŖ£ŗCa2+”¢Cu2+”¢Fe3+£»
¢Ū²»ÄÜČ·¶ØµÄĄė×ÓÓŠNO3-”¢Cl-£¬¹Ź“š°øĪŖ£ŗNO3-”¢Cl-£»
¢ÜÓŠ¹ŲĄė×Ó·½³ĢŹ½ÓŠ£ŗBa2++SO42-ØTBaSO4”ż£¬Ba2++CO32-ØTBaCO3”ż£¬BaCO3+2H+ØTBa2++H2O+CO2”ü£¬Ag++Cl-ØTAgCl”ż£®
¹Ź“š°øĪŖ£ŗBa2++SO42-ØTBaSO4”ż£¬Ba2++CO32-ØTBaCO3”ż£¬BaCO3+2H+ØTBa2++H2O+CO2”ü”ü£¬Ag++Cl-ØTAgCl”ż£®
µćĘĄ ±¾Ģāæ¼²éĄė×ӵļģŃéŅŌ¼°Ąė×Ó¹²“ęĪŹĢā£¬ĢāÄæÄŃ¶Č²»“ó£¬×¢Ņā³£¼ūĄė×ӵĊŌÖŹŗĶ¼ģŃé·½·Ø£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
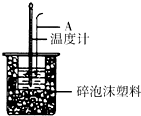 ĄūÓĆČēĶ¼×°ÖĆ²ā¶ØÖŠŗĶČȵďµŃé²½ÖčČēĻĀ£ŗ
ĄūÓĆČēĶ¼×°ÖĆ²ā¶ØÖŠŗĶČȵďµŃé²½ÖčČēĻĀ£ŗ| ĪĀ¶Č ŹµŃé“ĪŹż | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č t2/”ę | ||
| H2SO4 | NaOH | Ę½¾łÖµ | ||
| 1 | 25.0 | 25.2 | 28.5 | |
| 2 | 24.9 | 25.1 | 28.4 | |
| 3 | 25.5 | 26.5 | 31.8 | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
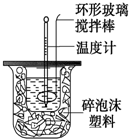 ²ā¶ØÖŠŗĶ·“Ó¦·“Ó¦ČȵďµŃé×°ÖĆČēĶ¼ĖłŹ¾£¬ŹµŃé½į¹ū²śÉśĘ«²īµÄŌŅņ²»æÉÄÜŹĒ£Ø””””£©
²ā¶ØÖŠŗĶ·“Ó¦·“Ó¦ČȵďµŃé×°ÖĆČēĶ¼ĖłŹ¾£¬ŹµŃé½į¹ū²śÉśĘ«²īµÄŌŅņ²»æÉÄÜŹĒ£Ø””””£©| A£® | ŹµŃéÖŠŹ¹ÓĆµÄ½Į°č°ō²ÄĮĻĪŖĢś | |
| B£® | ¶ĮČ”ĪĀ¶Č¼Ę¶ĮŹżŹ±£¬¶ĮČ”µÄŹĒ»ģŗĻČÜŅŗµÄ×īøßĪĀ¶Č | |
| C£® | ·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ | |
| D£® | ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŻĶČ” | B£® | ÕōĮó | C£® | ·ÖŅŗ | D£® | ÉųĪö |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1-±ū“¼ | B£® | 2£¬2-¶ž¼×»ł-1-±ū“¼ | ||
| C£® | 2£¬2£¬2-ČżäåŅŅ“¼ | D£® | ±½¼×“¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| Ėį | µēĄė·½³ĢŹ½ | µēĄėĘ½ŗā³£ŹżK |
| CH3COOH | CH3COOH?CH3COOH-+H+ | 1.76”Į10-5 |
| H2CO3 | H2CO3?H++HCO3-HCO3-?H++HCO32- | K1=4.31”Į10-7 K2=5.61”Į10-11 |
| H2S | H2S?H++HS-HS-?H++S2- | K1=9.1”Į10-8 K2=1.1”Į10-12 |
| H3PO4 | H3PO4?H++H2PO4-H2PO4-H++HPO42- HPO42-?H++PO43- | K1=7.52”Į10-3K2=6.23”Į10-8 K3=2.20”Į10-13 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com