
ЁОЬтФПЁПжаЙњЙХДњЮФЯзжаМЧдиСЫДѓСПЙХДњЛЏбЇЕФбаОПГЩЙћЃЌЁЖБОВнИйФПЁЗжаМЧдиЃКЁА(Л№вЉ)ФЫбцЯћ(KNO3)ЁЂСђЛЧЁЂЩМФОЬПЫљКЯЃЌвдЮЊЗщьняЅЛњжювЉепЁБЃЌЗДгІдРэЮЊЃКS+2KNO3+3C=K2S+N2Ёќ+3CO2ЁќЁЃ
(1)СђдзгЕФМлВуЕчзгХХВМЭМЮЊ_____ЃЌбЬЛЈШМЗХЙ§ГЬжаЃЌМидЊЫижаЕФЕчзгдОЧЈЕФЗНЪНЪЧ____ЃЌKЁЂSЁЂNЁЂOЫФжждЊЫиЕквЛЕчРыФмгЩДѓЕНаЁЕФЫГађЮЊ____ЁЃЩЯЪіЗДгІЩцМАЕФдЊЫижаЕчИКадзюДѓЕФЪЧ_____(ЬюдЊЫиЗћКХ)ЁЃ
(2)ЬМдЊЫиГ§ПЩаЮГЩГЃМћЕФбѕЛЏЮяCOЁЂCO2ЭтЃЌЛЙПЩаЮГЩC2O3(НсЙЙЪНЮЊ![]() )ЁЃC2O3гыЫЎЗДгІПЩЩњГЩВнЫс(HOOCЁЊCOOH)ЁЃ
)ЁЃC2O3гыЫЎЗДгІПЩЩњГЩВнЫс(HOOCЁЊCOOH)ЁЃ
ЂйC2O3жаЬМдзгЕФдгЛЏЙьЕРРраЭЮЊ______ЃЌCO2ЗжзгЕФСЂЬхЙЙаЭЮЊ_____ЁЃ
ЂкВнЫсгые§ЖЁЫс(CH3CH2CH2COOH)ЕФЯрЖдЗжзгжЪСПЯрВю2ЃЌЖўепЕФШлЕуЗжБ№ЮЊ101ЁцЁЂ-7.9ЁцЃЌЕМжТетжжВювьЕФзюжївЊдвђПЩФмЪЧ______ЁЃ
ЂлCOЗжзгжаІаМќгыІвМќИіЪ§БШЮЊ______ЁЃ
(3)ГЌбѕЛЏМиЕФОЇАћНсЙЙЭМШчЯТЃЌдђгыK+ЕШОрРыЧвзюНќЕФO2 - ИіЪ§ЮЊ_____ЃЌШєОЇАћВЮЪ§ЮЊdpmЃЌдђИУГЌбѕЛЏЮяЕФУмЖШЮЊ___gЁЄcmЃ3(гУКЌdЁЂNAЕФДњЪ§ЪНБэЪОЃЌЩшNAБэЪОАЂЗќМгЕТТоГЃЪ§ЕФжЕ)ЁЃ
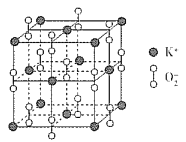
ЁОД№АИЁП![]() гЩИпФмСПзДЬЌдОЧЈЕНЕЭФмСПзДЬЌ NЃОOЃОSЃОK O sp2 жБЯпаЮ ВнЫсЗжзгжЎМфФмаЮГЩИќЖрЕФЧтМќ 2 : 1 6 284ЁС1030 /(NAЁЄd3) Лђ 2.84ЁС1032 /(NAЁЄd3)
гЩИпФмСПзДЬЌдОЧЈЕНЕЭФмСПзДЬЌ NЃОOЃОSЃОK O sp2 жБЯпаЮ ВнЫсЗжзгжЎМфФмаЮГЩИќЖрЕФЧтМќ 2 : 1 6 284ЁС1030 /(NAЁЄd3) Лђ 2.84ЁС1032 /(NAЁЄd3)
ЁОНтЮіЁП
ЃЈ1ЃЉЛљЬЌSдзгМлЕчзгХХВМЪНЮЊ3s23p3ЃЌНсКЯХнРћдРэЁЂКщЬиЙцдђЃЌМлЕчзгХХВМЭМЮЊ![]() ЃЛбЬЛЈШМЗХЙ§ГЬжаЃЌМидЊЫижаЕФЕчзггЩИпФмСПзДЬЌдОЧЈЕНЕЭФмСПзДЬЌЃЌвдЙтЕФаЮЪНЪЭЗХФмСПЃЛН№ЪєЕФЕквЛЕчРыФмаЁгкЗЧН№ЪєдЊЫиЕФЃЌNдзгдЊЫи2pФмМЖЮЊАыГфТњЮШЖЈзДЬЌЃЌNдЊЫиЕФЕквЛЕчРыФмИпгкOдЊЫиЕФЃЌЭЌжїзхздЩЯЖјЯТЕквЛЕчРыФмМѕаЁЃЌЙЪЕквЛЕчРыФмЃКNЃОOЃОSЃОKЃЛЭЌжмЦкздзѓЖјгвЕчИКаддіДѓЁЂЭЌжїзхздЩЯЖјЯТЕчИКадМѕаЁЃЌЙЪOЕФЕчИКадзюДѓЃЛ
ЃЛбЬЛЈШМЗХЙ§ГЬжаЃЌМидЊЫижаЕФЕчзггЩИпФмСПзДЬЌдОЧЈЕНЕЭФмСПзДЬЌЃЌвдЙтЕФаЮЪНЪЭЗХФмСПЃЛН№ЪєЕФЕквЛЕчРыФмаЁгкЗЧН№ЪєдЊЫиЕФЃЌNдзгдЊЫи2pФмМЖЮЊАыГфТњЮШЖЈзДЬЌЃЌNдЊЫиЕФЕквЛЕчРыФмИпгкOдЊЫиЕФЃЌЭЌжїзхздЩЯЖјЯТЕквЛЕчРыФмМѕаЁЃЌЙЪЕквЛЕчРыФмЃКNЃОOЃОSЃОKЃЛЭЌжмЦкздзѓЖјгвЕчИКаддіДѓЁЂЭЌжїзхздЩЯЖјЯТЕчИКадМѕаЁЃЌЙЪOЕФЕчИКадзюДѓЃЛ
ЃЈ2ЃЉЂйC2O3жаЬМдзгУЛгаЙТЖдЕчзгЁЂаЮГЩ3ИіІвМќЃЌдгЛЏЙьЕРЪ§ФПЮЊ3ЃЌCдзгВЩШЁsp2дгЛЏЃЛCO2ЗжзгжаЬМдзгУЛгаЙТЖдЕчзгЃЌМлВуЕчзгЖдЪ§ЮЊ2ЃЌЦфСЂЬхЙЙаЭЮЊжБЯпаЮЃЛ
ЂкВнЫсЗжзгКЌга2ИіЁАO-HЁБМќЃЌе§ЖЁЫсЗжзгКЌга1ИіЁАO-HЁБМќЃЌВнЫсЗжзгжЎМфаЮГЩИќЖрЕФЧтМќЃЌЙЪВнЫсЕФЗаЕуЙЪе§ЖЁЫсИпЕФЖрЃЛ
ЂлCOЗжзггыN2ЛЅЮЊЕШЕчзгЬхЃЌНсЙЙЯрЫЦЃЌЙЪCOЕФНсЙЙЪНЮЊCЁдOЃЌШ§МќКЌга1ИіІвМќЁЂ2ИіІаМќЃЌЙЪCOЗжзгжаІаМќгыІвМќИіЪ§БШЮЊ2ЃК1ЃЛ
ЃЈ3ЃЉвдЖЅЕуKЃЋбаОПЃЌЦфЦНУцЩЯгыЦфОрРызюНќЕФO2-га4ИіЃЌЩЯЗНКЭЯТЗНИїгавЛИіЃЌЙВга6ИіЁЃОЇАћжаKЃЋРызгЪ§ФП=8ЁС1/8+6ЁС1/2=4ЁЂO2ЃРызгЪ§ФП=12ЁС1/4+1=4ЃЌОЇАћжЪСП=4ЁС71/NAgЃЌОЇЬхУмЖШ=4ЁС71/NAgЁТЃЈdЁС10-10 cm ЃЉ3=4ЁС71/NAЁС(dЁС10-10)3 gЁЄcm-3=284ЁС1030 /(NAЁЄd3) Лђ 2.84ЁС1032 /(NAЁЄd3)ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГбаОПадбЇЯАаЁзщЭЌбЇШЁV LХЈЫѕЕФКЃЫЎЃЌгУЯТЭМЫљЪОзАжУФЃФтЙЄвЕЗЈЬсфхЁЃЛиД№ЯТСаЮЪЬтЃК
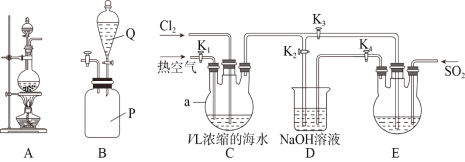
ЃЈ1ЃЉзАжУAПЩгУгкЪЕбщЪвжЦБИТШЦјЃЌЦфЗДгІдРэЮЊ________________________________(гУРызгЗНГЬЪНБэЪО)ЃЌзАжУBЪЧSO2жќЦјЦПЃЌдђQжаЕФШмвКЮЊ________________ЁЃ
ЃЈ2ЃЉфхЕФИЛМЏЙ§ГЬЃК
ЂйЭЈШыТШЦјгыЭЈШыШШПеЦјЕФЫГађЪЧЯШЭЈ______________ЃЌЭЈШыТШЦјЪБK1ЁЂK2ЁЂK3ЕФЙиЁЂПЊЗНЪНЪЧ___________________ЁЃ
ЂкзАжУDЕФзїгУЪЧ_______________________________ЃЌЭЈШыSO2ЪБзАжУEжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ______________________________________ЁЃ
ЃЈ3ЃЉфхЕФОЋжЦЃК
ЂйД§зАжУEжаBr-ШЋВПзЊЛЏЮЊЕЅжЪКѓЃЌдйРћгУЯТЭМжаЕФ____________(ЬюзжФИ)зАжУНјааеєСѓМДПЩЕУЕНДПфхЁЃ
ЂкМйЩшзюжеЕУЕНm gДПфхЃЌЪЕбщжафхЕФРћгУТЪЮЊb%ЃЌдђХЈЫѕКЃЫЎжаc(Br-)=______________.
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНсКЯдЊЫижмЦкБэЛиД№ЯТСаЮЪЬтЃК
(1)ЂйБэжаЪЕЯпЪЧдЊЫижмЦкБэЕФВПЗжБпНчЃЌЧыдкЭМ1жагУЪЕЯпВЙШЋдЊЫижмЦкБэЕФБпНч________
ЂкЧыЛГіН№ЪєгыЗЧН№ЪєЕФЗжНчЯп________
ЂлЧыдкЗНПђжа(ЭМ3)АДКЄдЊЫиЕФЪНбљаДГіhдЊЫидзгЕФЯрЙиаХЯЂ________
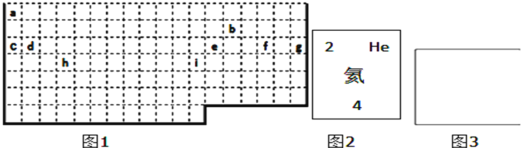
(2)XЁЂYЁЂZЪЧжмЦкБэжаЯрСкЕФШ§жжЖЬжмЦкдЊЫиЃЌXКЭYЭЌжмЦкЃЌYКЭZЭЌжїзхЃЌШ§жждЊЫидзгЕФзюЭтВуЕчзгЪ§жЎКЭЮЊ17ЃЌКЫФкжЪзгЪ§жЎКЭЮЊ31ЁЃ
ЂйXЪЧ______ЃЌ ZЪЧ______(ЬюаДдЊЫиУћГЦ)
ЂкX, Y, Z Ш§жждЊЫиЕФМђЕЅРызгАыОЖДѓаЁЫГађЪЧ_____________________________
ЂлШ§жждЊЫиаЮГЩЕФЕЅжЪбѕЛЏадзюЧПЕФЛЏбЇЪНЮЊ______
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖЬжмЦкдЊЫиXЁЂYЁЂZЁЂWдкдЊЫижмЦкБэжаЕФЯрЖдЮЛжУШчЭМЫљЪОЃЌЦфжаWдзгЕФжЪзгЪ§ЪЧЦфзюЭтВуЕчзгЪ§ЕФШ§БЖЃЌЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ![]()
A.зюМђЕЅЦјЬЌЧтЛЏЮяЕФШШЮШЖЈадЃК![]() X
X![]() W
W![]() Z
Z
B.зюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЕФЫсадЃК![]() W
W![]() Z
Z
C.дзгАыОЖЃК![]() Z
Z![]() Y
Y![]() X
X
D.дЊЫиXЁЂZЁЂWЕФзюИпе§МлЗжБ№гыЦфжїзхађЪ§ЯрЕШ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЛЗЮьЖўЯЉЃЈ![]() ЃЉЪЧживЊЕФгаЛњЛЏЙЄдСЯЃЌЙуЗКгУгкХЉвЉЁЂЯ№НКЁЂЫмСЯЕШЩњВњЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЉЪЧживЊЕФгаЛњЛЏЙЄдСЯЃЌЙуЗКгУгкХЉвЉЁЂЯ№НКЁЂЫмСЯЕШЩњВњЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉвбжЊЃК![]() (g) =
(g) = ![]() (g)+H2(g) ІЄH1=100.3 kJЁЄmol 1 ЂйЃЌH2(g)+ I2(g) =2HI(g) ІЄH2=11.0 kJЁЄmol 1 ЂкЃЌЖдгкЗДгІ
(g)+H2(g) ІЄH1=100.3 kJЁЄmol 1 ЂйЃЌH2(g)+ I2(g) =2HI(g) ІЄH2=11.0 kJЁЄmol 1 ЂкЃЌЖдгкЗДгІ![]() (g)+ I2(g) =
(g)+ I2(g) =![]() (g)+2HI(g) ЂлІЄH3=___________kJЁЄmol 1ЁЃ
(g)+2HI(g) ЂлІЄH3=___________kJЁЄmol 1ЁЃ
ЃЈ2ЃЉФГЮТЖШЯТЃЌЕШЮяжЪЕФСПЕФЕтКЭЛЗЮьЯЉЃЈ![]() ЃЉдкИеадШнЦїФкЗЂЩњЗДгІЂлЃЌЦ№ЪМзмбЙЮЊ105PaЃЌЦНКтЪБзмбЙдіМгСЫ30%ЃЌЛЗЮьЯЉЕФзЊЛЏТЪЮЊ___ЃЌИУЗДгІЕФЦНКтГЃЪ§Kp=____PaЁЃДяЕНЦНКтКѓЃЌгћдіМгЛЗЮьЯЉЕФЦНКтзЊЛЏТЪЃЌПЩВЩШЁЕФДыЪЉга_____ЃЈЬюБъКХЃЉЁЃ
ЃЉдкИеадШнЦїФкЗЂЩњЗДгІЂлЃЌЦ№ЪМзмбЙЮЊ105PaЃЌЦНКтЪБзмбЙдіМгСЫ30%ЃЌЛЗЮьЯЉЕФзЊЛЏТЪЮЊ___ЃЌИУЗДгІЕФЦНКтГЃЪ§Kp=____PaЁЃДяЕНЦНКтКѓЃЌгћдіМгЛЗЮьЯЉЕФЦНКтзЊЛЏТЪЃЌПЩВЩШЁЕФДыЪЉга_____ЃЈЬюБъКХЃЉЁЃ
AЃЎЭЈШыЖшадЦјЬх BЃЎЬсИпЮТЖШ CЃЎдіМгЛЗЮьЯЉХЈЖШ DЃЎдіМгЕтХЈЖШ EЃЎЪЙгУКЯЪЪДпЛЏМС
ЃЈ3ЃЉЛЗЮьЖўЯЉШнвзЗЂЩњОлКЯЩњГЩЖўОлЬхЃЌИУЗДгІЮЊПЩФцЗДгІЁЃВЛЭЌЮТЖШЯТЃЌШмвКжаЛЗЮьЖўЯЉХЈЖШгыЗДгІЪБМфЕФЙиЯЕШчЭМЫљЪОЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ____ЃЈЬюБъКХЃЉЁЃ

AЃЎT1ЃОT2 BЃЎaЕуЕФЗДгІЫйТЪаЁгкcЕуЕФЗДгІЫйТЪ
CЃЎaЕуЕФе§ЗДгІЫйТЪДѓгкbЕуЕФФцЗДгІЫйТЪ DЃЎbЕуЪБЖўОлЬхЕФХЈЖШЮЊ0.45 molЁЄL1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊAЪЧЛЏбЇЪЕбщЪвжазюГЃМћЕФгаЛњЮяЃЌЫќвзШмгкЫЎВЂгаЬиЪтЯуЮЖЃЛBЕФВњСППЩвдКтСПвЛИіЙњМвЪЏгЭЛЏЙЄЗЂеЙЕФЫЎЦНЃЌгаЙиЮяжЪЕФзЊЛЏЙиЯЕШчЭМЫљЪО![]() ВПЗжЗДгІЬѕМўЁЂВњЮяЪЁТд
ВПЗжЗДгІЬѕМўЁЂВњЮяЪЁТд![]() ЃК
ЃК
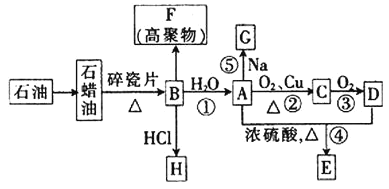
ЛиД№ЯТСаЮЪЬтЃК
![]() ЙЄвЕЩЯЃЌгЩЪЏгЭЛёЕУЪЏРЏгЭЕФЗНЗЈГЦЮЊ_________ЃЌгЩЪЏРЏгЭЛёЕУBЕФЗНЗЈГЦЮЊ__________ЁЃ
ЙЄвЕЩЯЃЌгЩЪЏгЭЛёЕУЪЏРЏгЭЕФЗНЗЈГЦЮЊ_________ЃЌгЩЪЏРЏгЭЛёЕУBЕФЗНЗЈГЦЮЊ__________ЁЃ
![]() ЂйОіЖЈЛЏКЯЮяAЕФЛЏбЇЬиадЕФдзгЭХЕФУћГЦЮЊ______________ЁЃ
ЂйОіЖЈЛЏКЯЮяAЕФЛЏбЇЬиадЕФдзгЭХЕФУћГЦЮЊ______________ЁЃ
Ђк![]() ЕНAЕФЗДгІРраЭЮЊ_______ЃЌAЕНEЕФЗДгІРраЭЮЊ____________ЁЃ
ЕНAЕФЗДгІРраЭЮЊ_______ЃЌAЕНEЕФЗДгІРраЭЮЊ____________ЁЃ
Ђл![]() ЕФЗжзгЪНЮЊ_________ЃЛFЕФНсЙЙМђЪНЮЊ___________ЁЃ
ЕФЗжзгЪНЮЊ_________ЃЛFЕФНсЙЙМђЪНЮЊ___________ЁЃ
![]() аДГіЯТСаЗДгІЕФЛЏбЇЗНГЬЪНЁЃ
аДГіЯТСаЗДгІЕФЛЏбЇЗНГЬЪНЁЃ
ЗДгІЂйЃК___________________________________________________ЃЛ
ЗДгІЂкЃК___________________________________________________ЃЛ
ЗДгІЂнЃК___________________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНёФъЪЧУХНнСаЗђЗЂЯждЊЫижмЦкТЩ150жмФъЁЃЯТБэЪЧдЊЫижмЦкБэЕФвЛВПЗжЃЌWЁЂXЁЂYЁЂZЮЊЖЬжмЦкжїзхдЊЫиЃЌWдзгзюЭтВуЕчзгЪ§ЪЧЦфФкВуЕчзгЪ§ЕФ3БЖЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧ
A.дзгАыОЖЃКW<X
B.Y ЕФзюИпМлбѕЛЏЮяЕФЫЎЛЏЮяЪЧЧПЫс
C.ЦјЬЌЧтЛЏЮяШШЮШЖЈадЃКZ<W
D.X ЕФЮЛжУЮЊЕкШ§жмЦкIVAзх
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЛЏКЯЮяXНігЩШ§жжЖЬжмЦкдЊЫизщГЩЃЌXИєОјПеЦјМгШШЃЌПЩЗжНтЮЊбЮAКЭЦјЬхBЃЌгаЙиИУЗДгІЕФЮяжЪзЊЛЛЁЂЯжЯѓКЭСПЕФЙиЯЕШчЯТЭМЫљЪОЁЃ

ЃЈ1ЃЉМьбщбЮAжабєРызгЕФЗНЗЈМАЯжЯѓЪЧ____________ЁЃ
ЃЈ2ЃЉЧыгУЫЋЧХЗЈЗжЮіВЂБэДяЛЏКЯЮяXЗжНтЪБЃЌЕчзгзЊвЦЕФЗНЯђКЭЪ§ФП____________ЁЃ
ЃЈ3ЃЉАзЩЋГСЕэCдкзЯЭтЙтееЩфЯТЛсЗЂЩњЗжНтЃЌЧыаДГіИУЗДгІЕФЛЏбЇЗНГЬЪН____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЁАТЬЩЋЪдМСЁБЫЋбѕЫЎПЩзїЮЊПѓвЕЗЯвКЯћЖОМСЃЌШчвЊЯћГ§ВЩПѓвЕЗЯвКжаЕФЧшЛЏЮяШчKCNЃЌЛЏбЇЗНГЬЪНЮЊЃКKCN+H2O2+KOHЁњK2CO3+NH3ЁќЁЃ
ЃЈ1ЃЉдкЛЏбЇЗНГЬЪНЩЯБъГіЕчзгзЊвЦЗНЯђКЭЪ§ФП___ЃЛ
ЃЈ2ЃЉЗДгІжаБЛбѕЛЏЕФдЊЫиЮЊ___ЃЌKOHЕФЕчзгЪНЮЊ___ЃЛ
ЃЈ3ЃЉNH3ЗжзгжаNЁЊHМќЮЊ__ЃЈЬюМЋадЛђЗЧМЋадЃЉЙВМлМќЃЛ
ЃЈ4ЃЉH2O2Ъєгк___ЃЈЬюЙВМлЛђРызгЃЉЛЏКЯЮяЃЛ
ЃЈ5ЃЉаДГіШмвКжаЗЂЩњИУЗДгІЪБЃЌЩњГЩЕФЛЏбЇМќРраЭ___ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com