

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
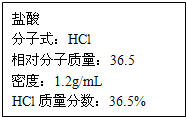 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺| 1000Vd |
| 22400+36.5V |
| 1000Vd |
| 22400+36.5V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
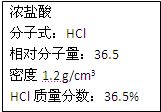 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��������2010��2011ѧ���һ10���¿���ѧ���� ���ͣ�058
| |||||||||||||||||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
|
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯������������ ��
A����Һ��HCl�����ʵ����� B����Һ��Ũ�� C����Һ��Cl-����Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.3 mol/Lϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ������ mL����Ũ����������ơ�
�����ƹ����У���Ҫʹ�õ������ǣ���д���ţ�________��
A���ձ����� B����Ͳ������������ C��1000 mL����ƿ���� D��������ƽ��
E��ҩ�ס��� F��500 mL����ƿ���� G����ͷ�ιܡ������� H��������
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ������������� ��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ��Լ30mL�����ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��500mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��
�������ƹ����У�����ʵ�������ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߵ�������
A������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
B����Һע������ƿǰû�лָ������¾ͽ��ж���
C������ʱ���ӿ̶��ߡ��������������� D��������ǰ����֪Ũ�ȵ�ϡ������ϴ����ƿ
��4�����ڱ�״���£���VLHCl��������1Lˮ�У�������Һ�ܶ�Ϊd g/mL�������Һ�����ʵ���Ũ��Ϊ ���� mol/L��

��5���ֽ�100mL0.5mol/L�������200mL0.1mol/LCuCl2��Һ��ϣ�����仯���Բ��ƣ�������Һ��Cl-�����ʵ���Ũ������������������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com