
| A£® | H3PO2ČÜÓŚĖ®µÄµēĄė·½³ĢŹ½ĪŖ£ŗH3PO2?H++H2PO2- | |
| B£® | H3PO2Óė¹żĮæNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗH3PO2+3OH-ØTPO23-+3H2O | |
| C£® | ½«H3PO2ČÜŅŗ¼ÓČėµ½ĖįŠŌøßĆĢĖį¼ŲČÜŅŗÖŠ£¬H3PO2µÄ»¹Ō²śĪļĪŖH3PO4 | |
| D£® | ÓƶčŠŌµē¼«µē½āNaH2PO2ČÜŅŗ£¬ĘäŃō¼«·“Ó¦Ź½ĪŖ£ŗ2H2O-4e-ØTO2”ü+4H+ |
·ÖĪö A£®Ņ»ŌŖČõĖįŌŚČÜŅŗÖŠ²æ·ÖµēĄė³öŅ»øöĒāĄė×Ó£»
B£®Ņ»ŌŖČõĖįÓėNaOH°“ÕÕĪļÖŹµÄĮæ1£ŗ1·“Ó¦£»
C£®H3PO2¾ßÓŠ»¹ŌŠŌ£¬Äܱ»øßĆĢĖį¼ŲŃõ»Æ£»
D£®H3PO2¾ßÓŠ½ĻĒæµÄ»¹ŌŠŌ£¬µē½āŹ±£¬Ńō¼«ÉĻH2PO2-Ź§µē×Ó£®
½ā“š ½ā£ŗA£®Ņ»ŌŖČõĖįŌŚČÜŅŗÖŠ²æ·ÖµēĄė³öŅ»øöĒāĄė×Ó£¬ŌņH3PO2ČÜÓŚĖ®µÄµēĄė·½³ĢŹ½ĪŖ£ŗH3PO2?H++H2PO2-£¬¹ŹAÕżČ·£»
B£®Ņ»ŌŖČõĖįÓėNaOH°“ÕÕĪļÖŹµÄĮæ1£ŗ1·“Ó¦£¬ĖłŅŌH3PO2Óė¹żĮæNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗH3PO2+OH-ØTH2PO2-+H2O£¬¹ŹB“ķĪó£»
C£®H3PO2¾ßÓŠ»¹ŌŠŌ£¬Äܱ»øßĆĢĖį¼ŲŃõ»Æ£¬Ōņ½«H3PO2ČÜŅŗ¼ÓČėµ½ĖįŠŌøßĆĢĖį¼ŲČÜŅŗÖŠ£¬H3PO2µÄŃõ»Æ²śĪļĪŖH3PO4£¬¹ŹC“ķĪó£»
D£®H3PO2¾ßÓŠ½ĻĒæµÄ»¹ŌŠŌ£¬µē½āŹ±£¬Ńō¼«ÉĻH2PO2-Ź§µē×Ó£¬ĖłŅŌÓƶčŠŌµē¼«µē½āNaH2PO2ČÜŅŗ£¬ĘäŃō¼«·“Ó¦Ź½ĪŖ£ŗH2PO2--4e-+2H2OØTH3PO4+3H+£¬¹ŹD“ķĪó£®
¹ŹŃ”A£®
µćĘĄ ±¾Ģāæ¼²éŃõ»Æ»¹Ō·“Ó¦”¢µēĄė·½³ĢŹ½µÄŹéŠ“”¢µē½āŌĄķµÄÓ¦ÓƵČÖŖŹ¶µć£¬Ć÷Č·ĪļÖŹµÄŠŌÖŹŹĒ½ā±¾Ģā¹Ų¼ü£¬ĄūÓƵē¼«·½³ĢŹ½µÄŹéŠ“·½·Ø”¢µēĄė·½³ĢŹ½ŹéŠ“¹ęŌņ”¢ŃĪĄąĖ®½āŌĄķ½ā“š¼“æÉ£¬ĢāÄæÄѶČÖŠµČ£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻõĖįŅųČÜŅŗ | B£® | ŹÆČļŹŌŅŗ | ||
| C£® | ŗ¬·ÓĢŖµÄĒāŃõ»ÆÄĘČÜŅŗ | D£® | µķ·Ūµā»Æ¼ŲŹŌÖ½ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ō×Ó°ė¾¶£ŗAl£¾Mg£¾Na£¾H | B£® | ČČĪČ¶ØŠŌ£ŗNH3£¾PH3£¾H2S£¾HCl | ||
| C£® | ĖįŠŌ£ŗHClO4£¾H2SiO3£¾H3PO4£¾H2CO3 | D£® | ŌŖĖŲ·Ē½šŹōŠŌ£ŗF£¾O£¾N£¾C |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{a}{A}$£ØA-N£©mol | B£® | $\frac{a}{A+2m}$ £ØA-N+m£©mol | C£® | $\frac{a}{A+2m}$£ØA-N£©mol | D£® | $\frac{a}{A+m}$£ØA-N+m£©mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 9g¼×»ł£Ø-CH3£©Ėłŗ¬ÓŠµÄµē×ÓŹżŹĒ10 NAøö | |
| B£® | 22.4LŅŅĶéÖŠŗ¬ÓŠµÄ¹²¼Ū¼üŹżĪŖ7NAøö | |
| C£® | ³£ĪĀĻĀ£¬14gŅŅĻ©ŗĶ±ūĻ©µÄ»ģŗĻĪļÖŠ×ÜŌ×ÓŹżĪŖ3NAøö | |
| D£® | 4.2g C3H6ÖŠŗ¬ÓŠµÄĢ¼Ģ¼Ė«¼üŹżŅ»¶ØĪŖ0.1NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé²Ł×÷¼°ĻÖĻó | ŹµŃé½įĀŪ |
| A | ĻņijČÜŅŗÖŠ¼ÓČėÓĆŃĪĖįĖį»ÆµÄĀČ»Æ±µČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É | øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠSO42- |
| B | ĻņijČÜŅŗÖŠ¼ÓČė2µĪKSCNČÜŅŗ£¬ČÜŅŗ²»ĻŌŗģÉ«£»ŌŁĻņČÜŅŗÖŠ¼ÓČė¼øµĪŠĀÖʵÄĀČĖ®£¬ČÜŅŗ±äĪŖŗģÉ« | øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠFe2+ |
| C | ½«Ä³ĘųĢåĶØČėĘ·ŗģČÜŅŗÖŠ£¬Ę·ŗģČÜŅŗĶŹÉ« | øĆĘųĢåŅ»¶ØŹĒSO2 |
| D | ĻņÉŁĮæijĪļÖŹµÄĻ”ČÜŅŗÖŠµĪ¼ÓĻ”ŃĪĖį£¬²śÉśĮĖÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢå | øĆĪļÖŹŅ»¶ØŹĒĢ¼ĖįŃĪ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
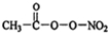 £ØPAN£©µČ¶ž“ĪĪŪČ¾Īļ£®
£ØPAN£©µČ¶ž“ĪĪŪČ¾Īļ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | »Æѧ·“Ó¦ÖŠŅ»¶ØÓŠ»Æѧ¼ü¶ĻĮŃ£¬Ķ¬Ź±Ņ²ÓŠ»Æѧ¼üŠĪ³É | |
| B£® | Ö»ÓŠ¹²¼Ū¼üµÄĪļÖŹŅ»¶ØŹĒ¹²¼Ū»ÆŗĻĪļ | |
| C£® | ŗ¬ÓŠŅõĄė×ӵĻÆŗĻĪļŅ»¶Øŗ¬ÓŠŃōĄė×Ó | |
| D£® | ·Ē½šŹōŌŖĖŲŗĶ·Ē½šŹōŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļ²»Ņ»¶ØŹĒĄė×Ó»ÆŗĻĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1-¼ŗĻ©”¢±½ | B£® | ŅŅĻ©”¢ŅŅČ² | C£® | ¼×±½”¢±½ | D£® | ¼ŗĶ锢ĪģĶé |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com