
���� A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��� �⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�������Ӧ���������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��AΪ̼Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪþԪ�أ�EΪCrԪ�أ�
��1��AΪ̼Ԫ�ء�BΪ��Ԫ�ء�CΪ��Ԫ�أ�ͬ����������ҵ�һ����������Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����������ͣ���Ԫ�ص�һ�����ܸ������ڵ�Ԫ�صģ����Ե�һ��������С�����˳��ΪC��O��N��
�ʴ�Ϊ��C��O��N��
��2��BΪ��Ԫ�أ����⻯��ΪNH3�������к���3��N-H����Nԭ����1�Թ¶Ե��Ӷԣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ռ乹��Ϊ�����ͣ�
�ʴ�Ϊ�������ͣ�sp3��
��3��������AC2��CO2��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O���ʴ�Ϊ��N2O��
��4��EΪCrԪ�أ�ԭ������Ϊ24��ԭ�Ӻ�����24�����ӣ���������Ų�ʽ�� 1s22s22p63s23p63d54s1��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��1s22s22p63s23p63d54s1��[Cr��NH3��4��H2O��2]Cl3��
��5��B������������Ӧ��ˮ����ΪHNO3��D�ĵ���ΪMg��HNO3ϡ��Һ��Mg��Ӧʱ��NԪ�ر���ԭ����ͼۣ�������NH4NO3��Mg������ΪMg��NO3��2����NH4NO3��Mg��NO3��2�Ļ�ѧ�������ֱ�Ϊx��y������ݵ���ת���غ���[5-��-3��]��x=2y������x��y=4��1���÷�Ӧ�Ļ�ѧ����ʽ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
���� ��Ŀ�ۺ��Խϴ��漰�ṹ����Խλ�ù�ϵ��Ԫ�������ɡ�����ʽ���������Ų�����������ӻ����ۡ����ӽṹ��������ԭ��Ӧ�ȣ��Ѷ��еȣ������ʽṹ���ۺ�����Ŀ���Ƕ�ѧ���ۺ������Ŀ��飬�⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ������������� | ʵ����� |
| A | ȡij±����������NaOH���Ҵ���Һ���ȣ�Ȼ����������ͨ�����Ը��������Һ�����Ը��������Һ��ɫ | ±������NaOH���Ҵ���Һ����������ϩ�� |
| B | �������������г���CO2��Ȼ�������ע��������ŨNaOH�������ý�����ס�ڣ��ޱ��������ݣ�һ��ʱ��ޱ������¹����� | NaOH��Һ��������CO2���ֿ����������Ӧ�������� |
| C | ȡ2mL 0.1mol•L-1 AgNO3��Һ������1mL 0.1mol•L-1 KSCN��Һ�����ú����ϲ���Һ�е���FeCl3��Һ����Һ��� | ��Һ����Ȼ��SCN- |
| D | ���ڿ��������չ��ijʺ�ɫ��ͭ˿���Ȳ���ʢ���Ҵ����Թ��У�ͭ˿��Ϊ��ɫ���������Σ��Թ��е�Һ���д̼�����ζ | �ڴ˷�Ӧ��ͭ˿������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
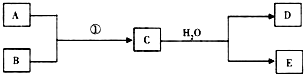 ��1����������Ϊ��ȼ��Ŀǰ60%��B���ǴӺ�ˮ����ȡ�ģ�����D����ʹʪ��ĺ�ɫʯ����ֽ������д��C��H2O��Ӧ�Ļ�ѧ����ʽMg3N2+6H2O=3Mg��OH��2+2NH3��������60��������˽�����D��Ϊȼ�ϵ�ص�ȼ��Դ���������飬�Ƴ�D-����ȼ�ϵ��ϵͳ���ܷ�ӦʽΪ��D+O2��A+H2O��δ��ƽ����д���˼���ȼ�ϵ�صĸ�����Ӧʽ��2NH3+6OH--6e-=N2+6H2O��
��1����������Ϊ��ȼ��Ŀǰ60%��B���ǴӺ�ˮ����ȡ�ģ�����D����ʹʪ��ĺ�ɫʯ����ֽ������д��C��H2O��Ӧ�Ļ�ѧ����ʽMg3N2+6H2O=3Mg��OH��2+2NH3��������60��������˽�����D��Ϊȼ�ϵ�ص�ȼ��Դ���������飬�Ƴ�D-����ȼ�ϵ��ϵͳ���ܷ�ӦʽΪ��D+O2��A+H2O��δ��ƽ����д���˼���ȼ�ϵ�صĸ�����Ӧʽ��2NH3+6OH--6e-=N2+6H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
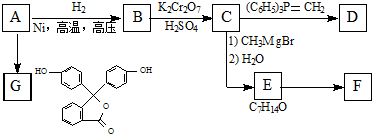
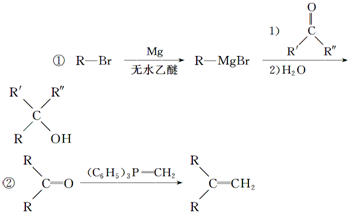 ��R��ʾ������R��R��ʾ�������⣩
��R��ʾ������R��R��ʾ�������⣩ ��
��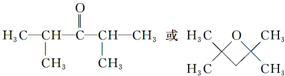 ����дһ�֣���
����дһ�֣���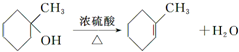 ��
��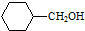 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C�TCH2+CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH��
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C�TCH2+CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
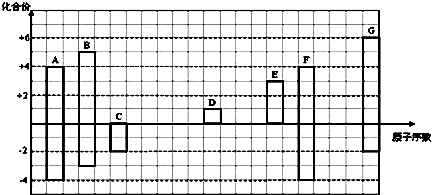
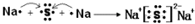 ��������ѧ������Ϊ���Ӽ�
��������ѧ������Ϊ���Ӽ� ��C��D�γɵľ���ǿ�����ԵĻ�����ĵ���ʽΪ
��C��D�γɵľ���ǿ�����ԵĻ�����ĵ���ʽΪ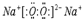

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʶ�����O2��ȼ�����ɹ������� | |
| B�� | ��Li��Cs���ܶ�Խ��Խ���۵�Խ��Խ�� | |
| C�� | ���ʶ����Ա�����ú���� | |
| D�� | ���ʶ���ǿ��ԭ������ˮ��Ӧ������ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� | ClO- | ClO2- | ClO3- | ClO4- |
| ����ṹ | ֱ�� | V�� | ������ | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H��Be��Bԭ�������������������� | B�� | P��S��ClԪ����������ϼ����ν��� | ||
| C�� | C��N��O��Fԭ�Ӱ뾶�������� | D�� | Li��Na��K��Rb�Ľ��������μ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
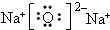 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com