

 £»ŅņĪŖ°ė³äĀś»ņČ«³”ĀśŹĒĪČ¶ØµÄ£¬ĖłŅŌCrµÄ×īĶā²ćµē×ÓŹĒ1£¬ÓėÖ®ĻąµČµÄ»¹ÓŠKŗĶCu£»CCl4”¢CS2ŹĒ·Ē¼«ŠŌ·Ö×Ó£¬øł¾ŻĻąĖĘĻąČÜŌĄķæÉÅŠ¶ĻDO2Cl2Ņ²ŹĒ·Ē¼«ŠŌ·Ö×Ó”£
£»ŅņĪŖ°ė³äĀś»ņČ«³”ĀśŹĒĪČ¶ØµÄ£¬ĖłŅŌCrµÄ×īĶā²ćµē×ÓŹĒ1£¬ÓėÖ®ĻąµČµÄ»¹ÓŠKŗĶCu£»CCl4”¢CS2ŹĒ·Ē¼«ŠŌ·Ö×Ó£¬øł¾ŻĻąĖĘĻąČÜŌĄķæÉÅŠ¶ĻDO2Cl2Ņ²ŹĒ·Ē¼«ŠŌ·Ö×Ó”£

 ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| ŌŖĖŲ±ąŗÅ | ŌŖĖŲŠŌÖŹ»ņŌ×Ó½į¹¹ |
| X | X+¾ßÓŠÓėNeŌ×ÓĻąĶ¬µÄµē×Ó²ć½į¹¹ |
| Y | ×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµÄŅ»°ė£¬Ęä×īøßÕż¼ŪÓė×īµĶøŗ¼ŪµÄ¾ų¶ŌÖµĻąµČ |
| Z | ³£ĪĀĻĀµ„ÖŹĪŖĖ«Ō×Ó·Ö×Ó£¬ĘäĒā»ÆĪļĖ®ČÜŅŗ³Ź¼īŠŌ |
| T | ŌŖĖŲµ„ÖŹµÄŃÕÉ«ŹĒ»ĘĀĢÉ«µÄĘųĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
| ŹµŃé·½°ø | ŹµŃéĻÖĻó |
| ¢ŁÓĆÉ°Ö½²ĮŗóµÄĆ¾“ųÓė·ŠĖ®·“Ó¦£¬ŌŁĻņ·“Ó¦ŗóČÜŅŗÖŠµĪ¼Ó·ÓĢŖ | (A)ø”ÓŚĖ®Ćę,ČŪ³ÉŅ»øöŠ”Ēņ,ŌŚĖ®ĆęÉĻĪŽ¶ØĻņŅʶÆ,ĖęÖ®ĻūŹ§,ČÜŅŗ±äŗģÉ« |
| ¢ŚĻņŠĀÖʵÄH2S±„ŗĶČÜŅŗÖŠµĪ¼ÓŠĀÖʵÄĀČĖ® | (B)²śÉśĘųĢå,æÉŌŚæÕĘųÖŠČ¼ÉÕ£¬ČÜŅŗ±ä³ÉĒ³ŗģÉ« |
| ¢ŪÄĘÓėµĪÓŠ·ÓĢŖŹŌŅŗµÄĄäĖ®·“Ó¦ | (C)·“Ó¦²»Ź®·ÖĒæĮŅ,²śÉśµÄĘųĢåæÉŅŌŌŚæÕĘųÖŠČ¼ÉÕ |
| ¢ÜĆ¾“ųÓė2 mol”¤L£1µÄŃĪĖį·“Ó¦ | (D)¾ēĮŅ·“Ó¦,²śÉśæÉČ¼ŠŌĘųĢå |
| ¢ŻĀĮĢõÓė2 mol”¤L£1µÄŃĪĖį·“Ó¦ | (E) Éś³Éµ»ĘÉ«³Įµķ |
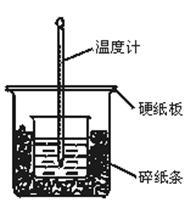
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| | ¢ńA | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | 0 |
| 1 | A | | ||||||
| 2 | | | | D | E | K | G | |
| 3 | B | | C | J | F | | H | I |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĖįŠŌ£ŗHClO4£¾H2SO4£¾H3PO4£¾H2SiO3 | B£®ĪČ¶ØŠŌ£ŗHF£¾H2O£¾H2S |
| C£®¼īŠŌ£ŗKOH£¾NaOH£¾Mg(OH)2 | D£®ČŪ·Šµć£ŗHI£¾HBr£¾HCl£¾HF |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®IB | B£®IIA | C£®IIB | D£®VIII |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®H2O2ÖŠŃõŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ-2 | B£®O2ÖŠŃõŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ-2 |
| C£®NaOHÖŠŃõŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ-1 | D£®Na2OÖŠŃõŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ-2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®100 | B£®95 | C£®88 | D£®80 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com