
ŹµŃéŹŅÓĆÉŁĮæµÄäåĖ®ŗĶ×ćĮæµÄŅŅ“¼Öʱø1,2”Ŗ¶žäåŅŅĶéµÄ×°ÖĆČēĻĀĶ¼ĖłŹ¾£ŗ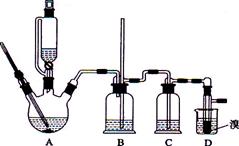
ÓŠ¹ŲŹż¾ŻĮŠ±ķČēĻĀ£ŗ
| | ŅŅ“¼ | 1,2-¶žäåŅŅĶé | ŅŅĆŃ |
| דĢ¬ | ĪŽÉ«ŅŗĢå | ĪŽÉ«ŅŗĢå | ĪŽÉ«ŅŗĢå |
| ĆÜ¶Č£Æg”¤ cm-3 | 0.79 | 2.2 | 0.71 |
| ·Šµć£Æ”ę | 78.5 | 132 | 34.6 |
| ČŪµć£Æ”ę | -l30 | 9 | -1l6 |
£Ø1£©ŹµŃéŹŅÖĘŅŅĻ©µÄ·½³ĢŹ½C2H5OH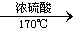 CH2=CH2”ü+H2O
CH2=CH2ӟ+H2O
£Ø2·Ö£©
£Ø2£©³¤µ¼¹ÜÖŠŅŗĆęÉĻÉż(2·Ö)
£Ø3£©c £Ø1·Ö£© ³żČ„æÉÄܲśÉśµÄĖįŠŌĘųĢå£ØÖ»ŅŖ“š³öSO2¼“æÉ£©£Ø2·Ö£©
£Ø4£©b £Ø2·Ö£©
£Ø5£©ÕōĮó £Ø2·Ö£©
£Ø6£©²śĘ·ČŪµćµĶ£¬¹ż¶ČĄäČ“»įÄż¹Ģ¶ų¶ĀČūµ¼¹Ü£Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ÉÕĘæAŹĒÖĘČ”ŅŅĻ©µÄ×°ÖĆ£¬·¢ÉśµÄ»Æѧ·½³ĢŹ½ĪŖC2H5OH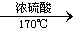 CH2=CH2”ü+H2O
CH2=CH2ӟ+H2O
£Ø2£©ČōD·¢Éś¶ĀČū£¬Ōņ»įµ¼ÖĀĢåĻµµÄĘųĢåĮ÷Ķز»³©£¬BÖŠµÄŃ¹ĒæŌö“󣬳¤µ¼¹ÜÖŠŅŗĆęÉĻÉż
£Ø3£©ŅŅ“¼ÓėÅØĮņĖį¹²ČČÖĘŅŅĻ©µÄ¹ż³ĢÖŠ»įÓŠ¶žŃõ»ÆĮņÉś³É£¬¶žŃõ»ÆĮņŅ²ÄÜŗĶäåĖ®·“Ó¦£¬ĖłŅŌÓ¦³żČ„£¬Ņņ“ĖŌŚ×°ÖĆCÖŠ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ£¬ÄæµÄŹĒ³żČ„²śÉśµÄ¶žŃõ»ÆĮņĘųĢå
£Ø4£©a”¢äåŌŚĖ®ÖŠµÄČܽā¶Č²»“󣬲»Ņ×ÓĆĖ®³żä壬“ķĪó£»b”¢ĒāŃõ»ÆÄĘČÜŅŗÓėäå·“Ó¦ĒŅÓė1,2”Ŗ¶žäåŅŅĶé²»»„ČÜ£¬æÉŅŌ³żäå£¬ÕżČ·£»c”¢µā»ÆÄĘĖäÓėäå·“Ó¦µ«Ķ¬Ź±Éś³ÉĮĖµ„ÖŹµā£¬“ķĪó£»d”¢ŅŅ“¼Óė1,2”Ŗ¶žäåŅŅĶ黄Čܲ»ÄܳżČ„ä壬“ķĪ󣬓š°øŃ”b”£
£Ø5£©ŅŅĆŃÓė1,2”Ŗ¶žäåŅŅĶ黄ČÜ£¬ÓĆÕōĮóµÄ·½·Ø³żČ„
£Ø6£©1,2”Ŗ¶žäåŅŅĶéµÄČŪµćµĶ£¬¹ż¶ČĄäČ“»įŹ¹²śĘ·Äż¹Ģ¶ų¶ĀČūŹŌ¹Ü
æ¼µć£ŗæ¼²éŹµŃéŹŅ1,2”Ŗ¶žäåŅŅĶéµÄÖʱøĖł·¢ÉśµÄ»Æѧ·“Ó¦”¢ĻÖĻ󔢳żŌÓµČÖŖŹ¶


 Ķ¬²½Į·Ļ°Ēæ»ÆĶŲÕ¹ĻµĮŠ“š°ø
Ķ¬²½Į·Ļ°Ēæ»ÆĶŲÕ¹ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø14·Ö£©Ä³Ń§ÉśĪŖ²ā¶ØijÉÕ¼īѳʷ֊NaOHµÄÖŹĮæ·ÖŹż£¬½ųŠŠČēĻĀŹµŃé£ØŅŃÖŖøĆѳʷ֊ŗ¬ÓŠÉŁĮæNa2CO3ŌÓÖŹ£©
a£®ŌŚ250mLµÄČŻĮæĘæÖŠ¶ØČŻ£¬ÅäÖĘ³É250mLÉÕ¼īČÜŅŗ”£
b£®ÓĆ¼īŹ½µĪ¶Ø¹ÜĮæČ”25.00mLÉÕ¼īČÜŅŗӌ׶ŠĪĘæÖŠ£¬¼ÓČė×ćĮæBaCl2ČÜŅŗŹ¹Na2CO3ĶźČ«×Ŗ±ä³ÉBaCO3ŗóµĪČė¼øµĪ·ÓĢŖÖøŹ¾¼Į£»
c£®ŌŚĢģĘ½ÉĻ×¼Č·³ĘČ”ÉÕ¼īѳʷ10.5g£¬ŌŚÉÕ±ÖŠÓĆÕōĮóĖ®Čܽā£»
d£®½«ĪļÖŹµÄĮæÅضČĪŖ1.000mol/LµÄ±ź×¼ĮņĖįČÜŅŗ×°ČėĖįŹ½µĪ¶Ø¹ÜÖŠ£¬µ÷½ŚŅŗĆę£¬¼ĒĻĀæŖŹ¼Ź±µÄ¶ĮŹżČ»ŗóæŖŹ¼µĪ¶Ø”£
e£®ŌŚ×¶ŠĪĘæĻĀµęŅ»ÕÅ°×Ö½£¬µĪ¶ØÖĮČÜŅŗĒ”ŗƱäĪŖĪŽÉ«ĪŖÖ¹£¬¼ĒĻĀ¶ĮŹż”£ŹŌĢīæÕ£ŗ
£Ø1£©ÕżČ·²Ł×÷²½ÖčµÄĖ³ŠņŹĒ£ØÓĆ×ÖÄøĢīæÕ£©_____”ś_____”ś_____”ś_____”ś_______.
£Ø2£©ĖįŹ½£Ø¼īŹ½£©µĪ¶Ø¹ÜŌŚŹ¹ÓĆĒ°Šč½ųŠŠµÄµŚŅ»²½²Ł×÷ŹĒ____________£¬ÖŠŃ§»ÆѧŹµŃé³£ÓĆŅĒĘ÷ÖŠŹ¹ÓĆĒ°ŗĶµĪ¶Ø¹ÜŹ¹ÓĆÓŠĻąĶ¬²Ł×÷µÄ²»Ķ¬Ąą²£Į§ŅĒĘ÷»¹ÓŠ ”¢_______”£
£Ø3£©ÖŲø“ÉĻŹöµĪ¶Ø²Ł×÷£¬¼ĒĀ¼Źż¾ŻČēĻĀ£ŗ
| ŹµŃ鱹ŗÅ | ±ź×¼ČÜŅŗ£ØH2SO4£©(aq) ÅØ¶Č£Ømol/L£© | µĪ¶ØĶź³ÉŹ±ŗÄĖį Ģå»żV£ØmL£© | “ż²āČÜŅŗ£ØNaOH£©(aq) Ģå»żV£ØmL£© |
| 1 | 1.000 | 11.00 | 25.00 |
| 2 | 1.000 | 12.04 | 25.00 |
| 3 | 1.000 | 12.18 | 25.00 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijĶ¬Ń§ÓūĢ½¾æŹ³Ę·Ģķ¼Ó¼Įļ§Ć÷·ÆNH4Al(SO4)2”¤12H2OøßĪĀ·Ö½āµÄĒéæö”£
£Ø1£©Ō¤²ā²śĪļ£ŗĻĀĮŠ¹ŲÓŚĘųĢå²śĪļµÄŌ¤²ā²»ŗĻĄķµÄŹĒ ”£
A£®NH3”¢N2”¢SO2”¢H2O B£®NH3”¢SO3”¢H2O
C£®NH3”¢SO2”¢H2O D£®NH3”¢N2”¢SO3”¢SO2”¢H2O
£Ø2£©¶ØŠŌ¼ģŃé£ŗČ”Ņ»¶ØĮæļ§Ć÷·Æ£¬Éč¼ĘĻĀĮŠŹµŃéĢ½¾æ²śĪļ”£
¢Ł°“Ķ¼Ź¾×é×°ŅĒĘ÷ŗó£¬Ź×ĻČ¼ģ²éÕūĢ××°ÖƵÄĘųĆÜŠŌ£¬²Ł×÷ŹĒ ”£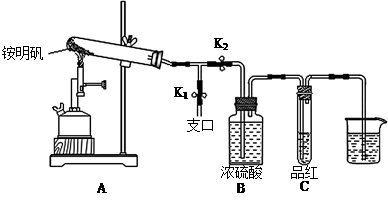
¢Ś¼Š×”Ö¹Ė®¼ŠK1£¬“ņæŖÖ¹Ė®¼ŠK2£¬ÓĆ¾Ę¾«ÅēµĘ³ä·Ö×ĘÉÕ”£ŹµŃé¹ż³ĢÖŠ£¬×°ÖĆAŗĶµ¼¹ÜÖŠĪ“¼ūŗģ×ŲÉ«ĘųĢ壻ŹŌ¹ÜCÖŠµÄĘ·ŗģČÜŅŗĶŹÉ«£»ŌŚÖ§æŚ“¦æɼģŃéµ½NH3£¬·½·ØŹĒ £»ŌŚ×°ÖĆAÓėBÖ®¼äµÄTŠĶµ¼¹ÜÖŠ³öĻÖ°×É«¹ĢĢ壬øĆ°×É«¹ĢĢåæÉÄÜŹĒ £ØČĪĢīŅ»ÖÖĪļÖŹµÄ»ÆѧŹ½£©”£
¢Ū·ÖĪöµĆ³ö×°ÖĆAŹŌ¹ÜÖŠ²ŠĮōµÄ°×É«¹ĢĢåŹĒĮ½ŠŌŃõ»ÆĪļ£¬Š“³öĖüČÜÓŚNaOHČÜŅŗµÄĄė×Ó·½³ĢŹ½ ”£
¢ÜĪŖĮĖ·ĄÖ¹µ¹Īü£¬ŹµŃé½įŹųŹ±±ŲŠėĻČ £ØĢī×ÖÄøŠņŗÅ£©£¬Č»ŗóĻØĆš¾Ę¾«ÅēµĘ”£
A£®Č”³öÉÕ±ÖŠµÄµ¼¹Ü B£®“ņæŖÖ¹Ė®¼ŠK1 C£®¹Ų±ÕÖ¹Ė®¼ŠK2
£Ø3£©·ÖĪöŗĶ½įĀŪ£ŗŹµŃéÖ¤Ć÷ĘųĢå²śĪļŹĒ£Ø1£©DÖŠµÄ5ÖÖĘųĢ唣ĻąĶ¬Ģõ¼žĻĀ²āµĆÉś³ÉN2ŗĶSO2µÄĢå»ż±ČŹĒ¶ØÖµ£¬V£ØN2£©£ŗV£ØSO2£©= ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¶ŌŹå¶”»ł±½·Ó æÉÓĆÓŚÉś²śÓĶČÜŠŌ·ÓČ©Ź÷Ö¬µČ”£ŹµŃéŹŅŅŌ±½·Ó”¢Źå¶”»łĀČ[(CH3)3CCl]µČĪŖŌĮĻÖʱø¶ŌŹå¶”»ł±½·ÓµÄŹµŃé²½ÖčČēĻĀ£ŗ
æÉÓĆÓŚÉś²śÓĶČÜŠŌ·ÓČ©Ź÷Ö¬µČ”£ŹµŃéŹŅŅŌ±½·Ó”¢Źå¶”»łĀČ[(CH3)3CCl]µČĪŖŌĮĻÖʱø¶ŌŹå¶”»ł±½·ÓµÄŹµŃé²½ÖčČēĻĀ£ŗ
²½Öč1£ŗ°“Ķ¼16×é×°ŅĒĘ÷£¬ŌŚXÖŠ¼ÓČė2.2 mLŹå¶”»łĀČ(¹żĮæ)ŗĶ1.41 g±½·Ó£¬½Į°čŹ¹±½·ÓĶźČ«Čܽā”£
²½Öč2£ŗĻņXÖŠ¼ÓČėĪŽĖ®AlCl3¹ĢĢå×÷“߻ƼĮ£¬²»¶Ļ½Į°č£¬ÓŠĘųĢå·Å³ö”£
²½Öč3£ŗ·“Ó¦»ŗŗĶŗó£¬ĻņXÖŠ¼ÓČė8 mLĖ®ŗĶ1 mLÅØŃĪĖį£¬¼“ÓŠ°×É«¹ĢĢåĪö³ö”£
²½Öč4£ŗ³éĀĖµĆµ½°×É«¹ĢĢ壬Ļ“µÓ£¬ÓĆŹÆÓĶĆŃÖŲ½į¾§£¬µĆ¶ŌŹå¶”»ł±½·Ó1.8 g”£
£Ø1£©ŅĒĘ÷XµÄĆū³ĘĪŖ ”£
£Ø2£©²½Öč2ÖŠ·¢ÉśÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£ČōøĆ·“Ó¦¹żÓŚ¼¤ĮŅ£¬æɲÉČ”µÄŅ»ÖÖ“ėŹ©ĪŖ ”£
£Ø3£©Ķ¼16ÖŠµ¹æŪĀ©¶·µÄ×÷ÓĆŹĒ ”£
£Ø4£©ŹµŃé½įŹųŗ󣬶Ō²śĘ·½ųŠŠ¹āĘ×¼ų¶Ø½į¹ūČēĻĀ”£ĘäÖŠŹōÓŚŗģĶā¹āĘ×µÄĘ×Ķ¼ŹĒ (Ģī×ÖÄø)”£
£Ø5£©±¾ŹµŃéÖŠ£¬¶ŌŹå¶”»ł±½·ÓµÄ²śĀŹĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
±½¼×ĖįŅŅõ„£ØC9H10O2£©±šĆūĪŖ°²Ļ¢ĻćĖįŅŅõ„”£ĖüŹĒŅ»ÖÖĪŽÉ«ĶøĆ÷ŅŗĢ壬²»ČÜÓŚĖ®£¬ÉŌÓŠĖ®¹ūĘųĪ¶£¬ÓĆÓŚÅäÖĘĻćĖ®Ļć¾«ŗĶČĖŌģ¾«ÓĶ£¬“óĮæÓĆÓŚŹ³Ę·¹¤ŅµÖŠ£¬Ņ²æÉÓĆ×÷ÓŠ»śŗĻ³ÉÖŠ¼äĢ壬ČܼĮµČ”£ĘäÖʱø·½·ØĪŖ£ŗ
ŅŃÖŖ£ŗ
| Ćū³Ę | Ļą¶Ō·Ö×ÓÖŹĮæ | ŃÕÉ«£¬×“Ģ¬ | ·Šµć(”ę) | ĆܶČ(g”¤cm-3) |
| ±½¼×Ėį* | 122 | ĪŽÉ«Ę¬×“¾§Ģå | 249 | 1.2659 |
| ±½¼×ĖįŅŅõ„ | 150 | ĪŽÉ«³ĪĒåŅŗĢå | 212.6 | 1.05 |
| ŅŅ“¼ | 46 | ĪŽÉ«³ĪĒåŅŗĢå | 78.3 | 0.7893 |
| »·¼ŗĶé | 84 | ĪŽÉ«³ĪĒåŅŗĢå | 80.8 | 0.7318 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
)µ„¾§¹čŹĒŠÅĻ¢²śŅµÖŠÖŲŅŖµÄ»ł“”²ÄĮĻ”£¹¤ŅµÉĻæÉÓĆ½¹ĢæÓė¶žŃõ»Æ¹čµÄ»ģŗĻĪļŌŚøßĪĀĻĀÓėĀČĘų·“Ӧɜ³ÉSiCl4ŗĶCO£¬SiCl4¾Ģį“æŗóÓĆĒāĘų»¹ŌµĆøß“æ¹č”£ŅŌĻĀŹĒŹµŃéŹŅÖʱøSiCl4µÄ×°ÖĆŹ¾ŅāĶ¼”£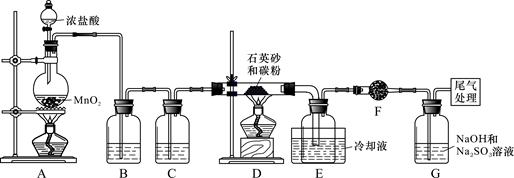
ŹµŃé¹ż³ĢÖŠ£¬ŹÆӢɰ֊µÄĢś”¢ĀĮµČŌÓÖŹŅ²ÄÜ×Ŗ»ÆĪŖĻąÓ¦ĀČ»ÆĪļ£¬SiCl4”¢AlCl3”¢FeCl3ÓöĖ®¾łŅ×Ė®½ā£¬ÓŠ¹ŲĪļÖŹµÄĪļĄķ³£Źż¼ūĻĀ±ķ£ŗ
| ĪļÖŹ | SiCl4 | AlCl3 | FeCl3 |
| ·Šµć/”ę | 57.7 | £ | 315 |
| ČŪµć/”ę | -70.0 | £ | £ |
| Éż»ŖĪĀ¶Č/”ę | £ | 180 | 300 |
| ŠņŗÅ | ²Ł ×÷ | æÉÄܳöĻÖµÄĻÖĻó | ½įĀŪ |
| ¢Ł | ĻņaŹŌ¹ÜÖŠµĪ¼Ó¼øµĪ ČÜŅŗ | ČōČÜŅŗĶŹÉ« | Ōņ¼ŁÉč1³ÉĮ¢ |
| ČōČÜŅŗ²»ĶŹÉ« | Ōņ¼ŁÉč2»ņ3³ÉĮ¢ | ||
| ¢Ś | ĻņbŹŌ¹ÜÖŠµĪ¼Ó¼øµĪ ČÜŅŗ | ČōČÜŅŗĶŹÉ« | Ōņ¼ŁÉč1»ņ3³ÉĮ¢ |
| ČōČÜŅŗ²»ĶŹÉ« | ¼ŁÉč2³ÉĮ¢ | ||
| ¢Ū | ĻņcŹŌ¹ÜÖŠµĪ¼Ó¼øµĪ ČÜŅŗ | | ¼ŁÉč3³ÉĮ¢ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²ŻĖį£ØH2C2O4£©ŹĒŅ»ÖÖÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ”£ĪŖĢ½¾æ²ŻĖįµÄÖĘČ”ŗĶ²ŻĖįµÄŠŌÖŹ£¬½ųŠŠČēĻĀŹµŃ锣
ŹµŃé¢ń£ŗŹµŃéŹŅÓĆĻõĖįŃõ»Æµķ·ŪĖ®½āŅŗ·ØÖʱø²ŻĖį£¬×°ÖĆČēĻĀĶ¼ĖłŹ¾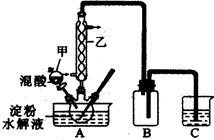
¢ŁŅ»¶ØĮæµÄµķ·ŪĖ®½āŅŗ¼ÓČėČż¾±ÉÕĘæÖŠ
¢ŚæŲÖĘ·“Ó¦ĪĀ¶Č55£60”ę£¬±ß½Į°č±ß»ŗĀżµĪ¼ÓŅ»¶ØĮæµÄ»ģŗĻĖį£Ø65£„µÄHNO3Óė98£„µÄH2SO4µÄÖŹĮæ±Č2©U1.25£©
¢Ū·“Ó¦3Š”Ź±£¬ĄäČ“£¬³éĀĖŗóŌŁÖŲ½į¾§µĆµ½²ŻĖį¾§Ģå
ĻõĖįŃõ»Æµķ·ŪĖ®½āŅŗµÄ·“Ó¦ĪŖ£ŗ
C6H12O6£«12HNO3 3H2C2O4£«9NO2”ü£«3NO”ü£«9H2O
3H2C2O4£«9NO2”ü£«3NO”ü£«9H2O
£Ø1£©ÉĻĶ¼ŹµŃé×°ÖĆÖŠŅĒĘ÷ŅŅµÄĆū³ĘĪŖ£ŗ________________________”£×°ÖĆBµÄ×÷ÓĆŹĒ ”£
£Ø2£©¼ģŃéµķ·ŪŹĒ·ńĖ®½āĶźČ«µÄ·½·Ø£ŗ______________________________________________”£ŹµŃé¢ņ£ŗĢ½¾æ²ŻĖįÓėĖįŠŌøßĆĢĖį¼ŲµÄ·“Ó¦
£Ø3£©Ļņ²ŻĖįČÜŅŗÖŠÖšµĪ¼ÓČėĮņĖįĖį»ÆµÄøßĆĢĖį¼ŲČÜŅŗŹ±£¬æɹŪ²ģµ½ČÜŅŗÓÉ×ĻŗģÉ«±äĪŖ½üŗõĪŽÉ«£¬Š“³öÉĻŹö·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ____________________________________________________”£
£Ø4£©Ń§Ļ°Š”×éµÄĶ¬Ń§·¢ĻÖ£¬µ±Ļņ²ŻĖįČÜŅŗÖŠÖšµĪ¼ÓČėĮņĖįĖį»ÆµÄøßĆĢĖį¼ŲČÜŅŗŹ±£¬ČÜŅŗĶŹÉ«×ÜŹĒĻČĀżŗóæģ”£ĪŖĢ½¾æĘäŌŅņ£¬Ķ¬Ń§ĆĒ×öĮĖČēĻĀ¶Ō±ČŹµŃ飻
ÓÉ“ĖÄćČĻĪŖČÜŅŗĶŹÉ«×ÜŹĒĻČĀżŗóæģµÄŌŅņŹĒ_________________________________________”£
£Ø5£©²ŻĖįŃĒĢśŌŚ¹¤ŅµÖŠÓŠÖŲŅŖ×÷ÓĆ£¬²ŻĖįæÉŅŌÖʱø²ŻĖįŃĒĢś£¬²½ÖčČēĻĀ£ŗ
³ĘČ”FeSO4”¤7H2O ¹ĢĢåÓŚŠ”ÉÕ±ÖŠ£¬¼ÓČėĖ®ŗĶÉŁĮæĻ”H2SO4ČÜŅŗĖį»Æ£¬¼ÓČČČܽā”£Ļņ“ĖČÜŅŗÖŠ¼ÓČėŅ»¶ØĮæµÄH2C2O4ČÜŅŗ£¬½«»ģŗĻČÜŅŗ¼ÓČČÖĮ·Š£¬²»¶Ļ½Į°č£¬ŅŌĆā±©·Š£¬“żÓŠ»ĘÉ«³ĮµķĪö³ö²¢³Įµķŗ󣬾²ÖĆ”£Ēć³öÉĻĒåŅŗ£¬ŌŁ¼ÓČėÉŁĮæĖ®£¬²¢¼ÓČČ£¬¹żĀĖ£¬³ä·ÖĻ“µÓ³Įµķ£¬¹żĀĖ£¬ÓƱūĶŖĻ“µÓ¹ĢĢåĮ½“Ī²¢ĮĄøÉ”£
¢ŁÉś³ÉµÄ²ŻĖįŃĒĢśŠč³ä·ÖĻ“µÓ³Įµķ£¬¼ģŃéŹĒ·ńĻ“µÓĶźČ«µÄ·½·ØŹĒ ”£
¢ŚÓƱūĶŖĻ“µÓ¹ĢĢåĮ½“ĪµÄÄæµÄŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
Õż¶”Č©ŹĒŅ»Öֻƹ¤ŌĮĻ”£Ä³ŹµŃ銔×éĄūÓĆČēĻĀ×°ÖĆŗĻ³ÉÕż¶”Č©”£
·¢ÉśµÄ·“Ó¦ČēĻĀ£ŗ
CH3CH2CH2CH2OH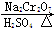 CH3CH2CH2CHO
CH3CH2CH2CHO
·“Ó¦ĪļŗĶ²śĪļµÄĻą¹ŲŹż¾ŻĮŠ±ķČēĻĀ£ŗ
| | ·Šµć/”ę | ĆܶČ/(g”¤cm£3) | Ė®ÖŠČܽāŠŌ |
| Õż¶”“¼ | 117.2 | 0.810 9 | Ī¢ČÜ |
| Õż¶”Č© | 75.7 | 0.801 7 | Ī¢ČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¶ŌĻõ»ł¼×±½ŹĒŅ½Ņ©”¢Č¾ĮĻµČ¹¤ŅµµÄŅ»ÖÖÖŲŅŖÓŠ»śÖŠ¼äĢ壬Ėü³£ŅŌÅØĻõĖįĪŖĻõ»Æ¼Į”¢ÅØĮņĖįĪŖ“߻ƼĮ£¬Ķعż¼×±½µÄĻõ»Æ·“Ó¦Öʱø”£
Ņ»ÖÖŠĀµÄÖʱø¶ŌĻõ»ł¼×±½µÄŹµŃé·½·ØŹĒ£ŗŅŌ·¢ŃĢĻõĖįĪŖĻõ»Æ¼Į£¬¹ĢĢåNaHSO4ĪŖ“߻ƼĮ(æÉŃ»·Ź¹ÓĆ)£¬ŌŚCCl4ČÜŅŗÖŠ£¬¼ÓČėŅŅĖįōū(ÓŠĶŃĖ®×÷ÓĆ)£¬45 ”ę·“Ó¦1 h”£·“Ó¦½įŹųŗ󣬹żĀĖ£¬ĀĖŅŗ·Ö±šÓĆ5% NaHCO3ČÜŅŗ”¢Ė®Ļ“ÖĮÖŠŠŌ£¬ŌŁ¾·ÖĄėĢį“æµĆµ½¶ŌĻõ»ł¼×±½”£
(1)ÉĻŹöŹµŃéÖŠ¹żĀĖµÄÄæµÄŹĒ_____________________________________”£
(2)ĀĖŅŗŌŚ·ÖŅŗĀ©¶·ÖŠĻ“µÓ¾²ÖĆŗó£¬ÓŠ»ś²ć“¦ÓŚ________²ć(Ģī”°ÉĻ”±»ņ”°ĻĀ”±)£»·ÅŅŗŹ±£¬Čō·¢ĻÖŅŗĢåĮ÷²»ĻĀĄ“£¬ĘäæÉÄÜŌŅņ³ż·ÖŅŗĀ©¶·»īČū¶ĀČūĶā£¬»¹ÓŠ_________________________________________________________”£
(3)ĻĀĮŠøų³öĮĖ“߻ƼĮÖÖĄą¼°ÓĆĮæ¶Ō¼×±½Ļõ»Æ·“Ó¦Ó°ĻģµÄŹµŃé½į¹ū”£
| “߻ƼĮ |  | Ļõ»Æ²śĪļÖŠø÷ÖÖŅģ¹¹ĢåÖŹĮæ·ÖŹż(%) | ×ܲśĀŹ(%) | ||
| ¶ŌĻõ»ł¼×±½ | ĮŚĻõ»ł¼×±½ | ¼äĻõ»ł¼×±½ | |||
| ÅØH2SO4 | 1.0 | 35.6 | 60.2 | 4.2 | 98.0 |
| 1.2 | 36.5 | 59.5 | 4.0 | 99.8 | |
| NaHSO4 | 0.15 | 44.6 | 55.1 | 0.3 | 98.9 |
| 0.25 | 46.3 | 52.8 | 0.9 | 99.9 | |
| 0.32 | 47.9 | 51.8 | 0.3 | 99.9 | |
| 0.36 | 45.2 | 54.2 | 0.6 | 99.9 | |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com