
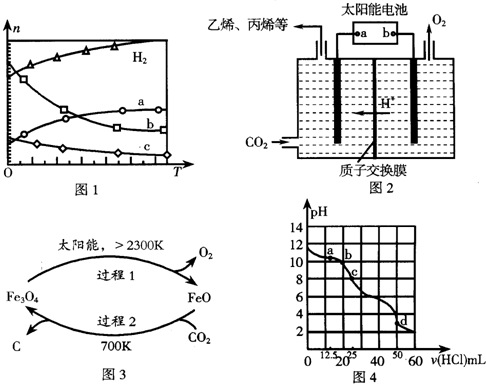
���� ��1�������߱仯��֪�����¶����ߣ����������ʵ��������࣬˵�������¶�ƽ�������ƶ���������Ӧ���ȣ����ݷ�Ӧ�������������ʵ����仯����Ӧ�м������Ĺ�ϵ��֪��aΪCO2�ı仯���ߣ�ˮ�ļ���������ϩ�ļ�������4�����ݴ��ж�c���ߣ��¶����ߣ����ȷ����ƽ�ⳣ�����ݴ��жϣ�
��2��̫���ܵ���й���ת��Ϊ���ܣ����ǿ���ԵĶ�����̼ˮ��Һ�õ���ϩ������ת��Ϊ��ѧ�ܣ�������̼�����ӵ�Դa���ĵ缫�ϵõ��ӷ�����ԭ��Ӧ������ϩ��aΪ��Դ������bΪ��Դ�������������ĵ缫��ӦʽΪ��3CO2+18H++18e-=C3H6+6H2O��
��3������ͼ֪��������Ӧ������2�з�����Ӧ�Ļ�ѧ����ʽΪ6FeO��S��+CO2$\frac{\underline{\;700K\;}}{\;}$2Fe3O4��S��+C������1�з�����Ӧ�Ļ�ѧ����ʽΪ2Fe3O4 $\frac{\underline{\;��2300\;}}{\;}$6FeO+O2����ÿ����2molFe3O4ת�Ƶ��ӵ����ʵ���Ϊ4mol������ÿ����1molFe3O4ת�Ƶ��ӵ����ʵ���Ϊ2mol��
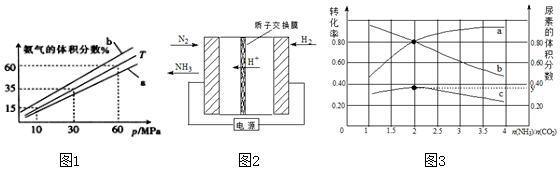
��4��һ����CO2����NaOH������ǡ�õõ�25mL0.1000mol/LNa2CO3��Һ���ڳ�������0.1000mol/L�����������еζ���c��ʱn��HCl��=0.1mol/L��0.025L=0.0025mol��ǡ����ȫ��Ӧ���ɵ����ʵ�����NaHCO3��NaCl����������Ũ�ȴ�СΪ��c��Na+����c��Cl-����c��HCO3-����c��CO32-����c��OH-����c��H+����
��5�������Ȼ�ѧ����ʽ��˹���ɼ����+��-�۵õ�CO2��NH3�ϳ����غ�Һ̬ˮ���Ȼ�ѧ��Ӧ����ʽ��
��� �⣺��1�������߱仯��֪�����¶����ߣ����������ʵ��������࣬˵�������¶�ƽ�������ƶ���������Ӧ���ȣ���H��0�������¶����ߣ����������ʵ��������࣬������Ϊ��Ӧ�����һ�������������ΪCO2���ɼ�������ϵ��֪bΪˮ��cΪC2H4�ı仯���ߣ������¶ȵ����ߣ��÷�Ӧ���淴Ӧ�����ƶ������Է�Ӧ�Ļ�ѧƽ�ⳣ����С��
�ʴ�Ϊ������C2H4����С��
��2��̫���ܵ���й���ת��Ϊ���ܣ����ǿ���ԵĶ�����̼ˮ��Һ�õ���ϩ������ת��Ϊ��ѧ�ܣ�������̼�����ӵ�Դa���ĵ缫�ϵõ��ӷ�����ԭ��Ӧ������ϩ��aΪ��Դ������bΪ��Դ�������������ĵ缫��ӦʽΪ��3CO2+18H++18e-=C3H6+6H2O��
�ʴ�Ϊ��a��3CO2+18H++18e-=C3H6+6H2O��
��3������ͼ֪��������Ӧ������2�з�����Ӧ�Ļ�ѧ����ʽΪ6FeO��S��+CO2$\frac{\underline{\;700K\;}}{\;}$2Fe3O4��S��+C������1�з�����Ӧ�Ļ�ѧ����ʽΪ2Fe3O4 $\frac{\underline{\;��2300\;}}{\;}$6FeO+O2����ÿ����2molFe3O4ת�Ƶ��ӵ����ʵ���Ϊ4mol������ÿ����1molFe3O4ת�Ƶ��ӵ����ʵ���Ϊ2mol��
�ʴ�Ϊ��6FeO��S��+CO2$\frac{\underline{\;700K\;}}{\;}$2Fe3O4��S��+C��2mol��
��4��һ����CO2����NaOH������ǡ�õõ�25mL0.1000mol/LNa2CO3��Һ���ڳ�������0.1000mol/L�����������еζ���c��ʱn��HCl��=0.1mol/L��0.025L=0.0025mol��ǡ����ȫ��Ӧ���ɵ����ʵ�����NaHCO3��NaCl����������Ũ�ȴ�СΪ��c��Na+����c��Cl-����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��Cl-����c��HCO3-����c��CO32-����c��OH-����c��H+����
��5����2NH3��g��+CO2��g��=NH2CO2 NH4��s����H=+l59.5kJ•mol-1
��NH2CO2NH4��s��=CO��NH2��2��s��+H2O��g����H=-116.5kJ•mol-1
��H2O��l��=H2O��g����H=+44.0kJ•mol-1
���Ȼ�ѧ����ʽ��˹���ɼ����+��-�۵õ�CO2��NH3�ϳ����غ�Һ̬ˮ���Ȼ�ѧ��Ӧ����ʽΪ2NH3��g��+CO2��g��=CO��NH2��2��s��+H2O��l����H=-87.0KJ/mol��
�ʴ�Ϊ��2NH3��g��+CO2��g��=CO��NH2��2��s��+H2O��l����H=-87.0KJ/mol��
���� ���⿼�黯ѧƽ��ͼ��ԭ��غ͵���ԭ����������ԭ��Ӧ�йؼ��㡢����Ũ�ȴ�С�ȽϺ�˹���ɵ�Ӧ�õȣ���4���йؼ����жϸ�����Һ�����ʣ��ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��25mL��ʽ�ζ�����ȡ���������Һ�����Ϊ16.60mL | |
| B�� | �ñ�NaOH��Һ�ζ�δ֪Ũ�����ᣬ��ȥNaOH��Һ20.50mL | |
| C�� | ��10mL��Ͳ��ȡ8.25mL���� | |
| D�� | ����ͨpH��ֽ���ij��ҺpHΪ3.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ�ӵĽṹʾ��ͼ��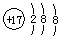 | B�� | �Ȼ�þ�ĵ���ʽ��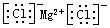 | ||
| C�� | N2�ĵ���ʽ�� | D�� | �Ȼ�����ӵ��γɹ��̣� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�¶�/K | 298 | 398 | 498 | �� |
| ƽ�ⳣ����K�� | 4.1��105 | K1 | K2 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
| ���� | A | B | C | D | E |
| ��������� ��Ӧˮ������ȶ��� | �ѷֽ� | �ֽܷ� | �ֽܷ� | �ֽܷ� | �ֽܷ� |
| �������� | ��ˮ���ҷ�Ӧ | ����������ˮ | ����ǿ������Һ | ���������Ũ���� | ����Ũ��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ��ѧ�� | C-H | C-C | C�TC | H-H |
| ����/kJ•mol-1 | 412 | 348 | 612 | 436 |
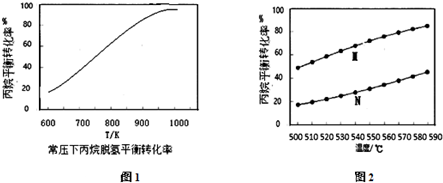
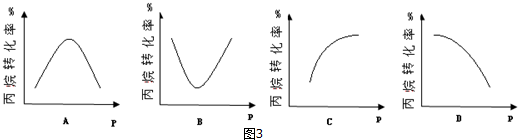
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
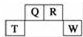 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com