
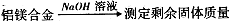 ӣ
ӣ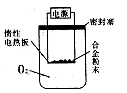
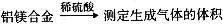 ӣ
ӣ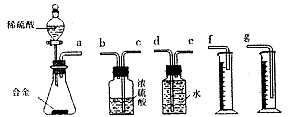
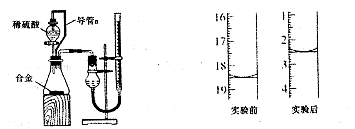
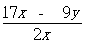
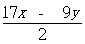 ,ŌņĆ¾µÄÖŹĮæ·ÖŹżĪŖ”£
,ŌņĆ¾µÄÖŹĮæ·ÖŹżĪŖ”£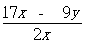


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®MnO2 | B£®Fe£³O£“ | C£®Cr2O3 | D£®V2O5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
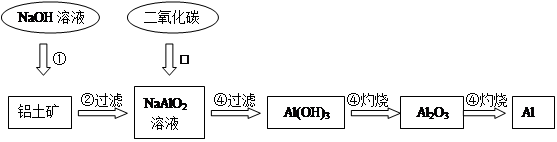
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

 4AlCl3+3O2
4AlCl3+3O2 AlCl3+X”ü£¬ĪŖČ·¶ØĘųĢåXŹĒ·ńŹĒ»ģŗĻĘųĢ壬ijĶ¬Ń§½«XŅĄ“ĪĶعż×ĘČȵÄŃõ»ÆĶŗĶ³ĪĒåµÄŹÆ»ŅĖ®£¬ŌŁøł¾ŻĻÖĻóÅŠ¶Ļ”£øĆ²Ł×÷ŹĒ·ńÕżČ·£æ£ØĢīÕżČ·”¢²»ÕżČ·»ņĪŽ·ØÅŠ¶Ļ£© £¬ĒėĖµĆ÷ĄķÓÉ ”£
AlCl3+X”ü£¬ĪŖČ·¶ØĘųĢåXŹĒ·ńŹĒ»ģŗĻĘųĢ壬ijĶ¬Ń§½«XŅĄ“ĪĶعż×ĘČȵÄŃõ»ÆĶŗĶ³ĪĒåµÄŹÆ»ŅĖ®£¬ŌŁøł¾ŻĻÖĻóÅŠ¶Ļ”£øĆ²Ł×÷ŹĒ·ńÕżČ·£æ£ØĢīÕżČ·”¢²»ÕżČ·»ņĪŽ·ØÅŠ¶Ļ£© £¬ĒėĖµĆ÷ĄķÓÉ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŹÆ»ŅĖ® | B£®ĒāŃõ»ÆÄĘČÜŅŗ | C£®ĮņĖį | D£®°±Ė® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ś | B£®¢Ł | C£®¢Ū | D£®¢Ł”¢¢Ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 ”£¹ŲÓŚøĆĪļÖŹµÄĖµ·ØÕżČ·µÄŹĒ£Ø £©
”£¹ŲÓŚøĆĪļÖŹµÄĖµ·ØÕżČ·µÄŹĒ£Ø £©| A£®øĆĪļÖŹŹōÓŚĮ½ŠŌĒāŃõ»ÆĪļ |
| B£®øĆĪļÖŹŹĒAl£ØOH£©3ŗĶNa2CO3µÄ»ģŗĻĪļ |
C£®1 mol NaAl£ØOH£©2CO3×ī¶ąæÉĻūŗÄ3 mol H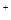 |
| D£®øĆŅ©¼Į²»ŹŹŗĻÓŚĪøĄ£Ńń»¼Õß·žÓĆ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com