��2012?����һģ��FeSO
4?7H
2O�㷺����ҽҩ��ҵ����������FeSO
4?7H
2O��ʵ�����Ʊ�����ͼ�������������������գ�
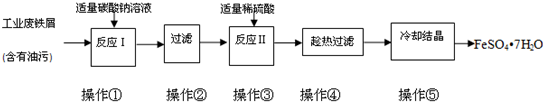
��1����������̼������Һ�ܳ�ȥ���ۣ�ԭ����
CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-
CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-
�������ӷ���ʽ��ʾ����
��2���������ʷ��뷽����ԭ���������ͬ����
c
c
���������ĸ����
a������ b����Һ
c������ d���ؽᾧ
��3��FeSO
4?7H
2O��ijЩ��Ѫ������Ҫ�ɷ֣�ʵ������Ϊ�ⶨij��Ѫ������Ԫ�صĺ���������������ʵ�飺
������100mL 1.00��10
-2mol?L
-1��KMnO
4��Һ�����õ���������ƽ���ձ�����ͷ�ιܼ�
100mL����ƿ��������
100mL����ƿ��������
�����ƹ���������˵���У���ȷ����
bd
bd
���������ĸ����
a��KMnO
4����ˮ�����ȣ�����ֱ��������ƿ���ܽ�
b������ƿϴ�Ӻ�����T��ֱ������ʵ��
c�����ݺ�ҡ�ȣ���Һ����ڿ̶��ߣ��ټ�ˮ����Һ����͵���̶�����ƽ
d���������ʱ��ˮ�����̶��߱�����������
��ȡ2.0g�˸ò�Ѫ�������Һ������Ԥ������ʹ���е���Ԫ��ȫ����Fe
2+���������ƺõı�KMnO
4��Һ�����������½���������ԭ�ζ�����Ӧ�����ӷ���ʽ�ǣ�5Fe
2++MnO
4-+8H
+�T5Fe
3++Mn
2++4H
2O�����еζ�ʱ����KMnO
4��ҺӦ��ʢ����
��ʽ
��ʽ
�����ʽ����ʽ��֮һ���ζ����У�ԭ����
KMnO4��Һ��ǿ�������ܸ�ʴ��
KMnO4��Һ��ǿ�������ܸ�ʴ��
��
�۵ζ�����ʱ������Ũ��Ϊ1.00��10
-2mol?L
-1�ı�KMnO
4��Һ26.00mL����ò�Ѫ������Ԫ�صĺ���Ϊ
3.64
3.64
%��




 ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�
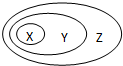 ��2012?����һģ���±��������ʻ�����Ĵ�����ϵ������ͼ��ʾ��ϵ���ǣ�������
��2012?����һģ���±��������ʻ�����Ĵ�����ϵ������ͼ��ʾ��ϵ���ǣ�������