

 µÄŃÕÉ«Ņ²±ä³ÉĪŽÉ«£¬ĒėÄć°ļĖū¶Ō²śÉśµÄĘųĢå½ųŠŠĢ½¾æ£ŗ
µÄŃÕÉ«Ņ²±ä³ÉĪŽÉ«£¬ĒėÄć°ļĖū¶Ō²śÉśµÄĘųĢå½ųŠŠĢ½¾æ£ŗ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®H2SŗĶNH3¾łŹĒ¼Ūµē×Ó×ÜŹżĪŖ8µÄ¼«ŠŌ·Ö×Ó |
| B£®HS£ŗĶHCl¾łŹĒŗ¬Ņ»øö¼«ŠŌ¼üµÄ18µē×ÓĮ£×Ó |
| C£®CH2Cl2ŗĶCCl4¾łŹĒĖÄĆęĢå¹¹ŠĶµÄ·Ē¼«ŠŌ·Ö×Ó |
| D£®1 mol DOÖŠŗ¬ÖŠ×Ó”¢ÖŹ×Ó”¢µē×Óø÷10 NA(NAĪŖ°¢·ü¼ÓµĀĀŽ³£Źż) |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÓÅĻȵ„¶ĄÕ¼¾Ż²»Ķ¬¹ģµĄ£¬ĒŅ×ŌŠż·½ĻņĻąĶ¬ |
| B£®ÓÅĻȵ„¶ĄÕ¼¾Ż²»Ķ¬¹ģµĄ£¬ĒŅ×ŌŠż·½ĻņĻą·“ |
| C£®×ŌÓÉÅä¶Ō£¬ÓÅĻČÕ¼¾ŻĶ¬Ņ»¹ģµĄ£¬ĒŅ×ŌŠż·½ĻņĻąĶ¬ |
| D£®×ŌÓÉÅä¶Ō£¬ÓÅĻČÕ¼¾ŻĶ¬Ņ»¹ģµĄ£¬ĒŅ×ŌŠż·½ĻņĻą·“ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
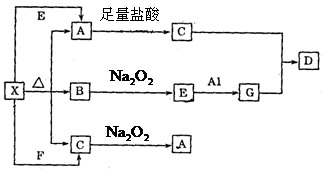
 ³öGÓė¹żĮæµÄC·“Ӧɜ³ÉDµÄĄė×Ó·½³ĢŹ½£ŗ £»
³öGÓė¹żĮæµÄC·“Ӧɜ³ÉDµÄĄė×Ó·½³ĢŹ½£ŗ £» AµÄĄė×Ó·½³ĢŹ½£ŗ £»
AµÄĄė×Ó·½³ĢŹ½£ŗ £»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø²æ·Ö·“Ó¦ĪļÓė²śĪļŅŃĀŌČ„£©£ŗ
¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø²æ·Ö·“Ó¦ĪļÓė²śĪļŅŃĀŌČ„£©£ŗ
 ӣ
”£ ×ӵďµŃé²Ł×÷ŹĒ ”£
×ӵďµŃé²Ł×÷ŹĒ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®a£c£½m£n | B£®a£b£½n£m | C£®c£d£½m£«n | D£®b£d£½n£«m |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

| | Óŵć | ȱµć |
| Ź¹ÓĆÅØĻõĖį | ·“Ó¦ĖŁĀŹæģ | ĖįŗĽĻ“󣬲śÉśNOxµÄĮæ½Ļ¶ą |
| Ź¹ÓĆĻ”ĻõĖį | | |
 £»
£» ”£
”£ Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ ”£
Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com