
����Ŀ�������̿��������������ӵ�صĸ������ϡ��������·�Ӧ�Ƶ������̣�4Mn(NO3)2��6H2O+26(CH3CO)2O ��4(CH3COO)3Mn +8HNO2+ 3O2��+40CH3COOH
��1��Mn3+��̬��������Ų�ʽΪ______��
��2��NO�е�ԭ�ӹ�����ӻ�������______��
��3����HNO2��Ϊ�ȵ������һ�������ӵĻ�ѧʽΪ______��
��4�������[Mn(CH3OH)6]2+���ṩ�¶Ե��ӵ�ԭ����______��
��5��CH3COOH����H2O����Ȼ��ܵ�ԭ�����Ƕ��Ǽ��Է������______��
��6��þ���Ͻ����´����һ�ִ��Ʋ��ϣ��侧��Ϊ�����ṹ����ͼ��ʾ����ͼ��ԭ��λ�ڶ�������ġ��þ�����ÿ����ԭ����Χ���������þԭ����ĿΪ______��

���𰸡� [Ar]3d4 sp2 HCOO- O CH3COOH������H2O���Ӽ���γ���� 8
��������(1)��Ϊ25��Ԫ�أ���������Ų�ʽΪ1s22s22p63s23p63d54s2����Mn3+��̬��������Ų�ʽΪ1s22s22p63s23p63d4��[Ar]3d4���ʴ�Ϊ��1s22s22p63s23p63d4��[Ar]3d4��
(2)NO��Nԭ�ӵļ۲���Ӷ���=3+![]() =3����ԭ�Ӳ���sp2�ӻ����ʴ�Ϊ��sp2��
=3����ԭ�Ӳ���sp2�ӻ����ʴ�Ϊ��sp2��
(3)��HNO2��Ϊ�ȵ������һ��������ΪHCOO-���ʴ�Ϊ��HCOO-��
(4)�����[Mn(CH3OH)6]2+�е�����ԭ��ΪMn������ΪCH3OH���ṩ�¶Ե��ӵ�ԭ�����ǻ��е�Oԭ�ӣ��ʴ�Ϊ��O��
(5)CH3COOH������H2O���Ӷ��Ǽ��Է��ӣ�����CH3COOH������H2O���Ӽ���γ������ʹ��CH3COOH����H2O����Ȼ��ܣ��ʴ�Ϊ��CH3COOH������H2O���Ӽ���γ������
(6)����þ���Ͻ�ľ����ṹ��֪��ͼ��ԭ��λ�ڶ�������ģ�����þԭ�Ӷ��������2�����ģ���ԭ��λ������2�����ģ�ÿ����ԭ����Χ���������þԭ����8��������ͬһ�����4�������4�����ģ������Ϊ������Խ��߳��ȵ�һ�룬�ʴ�Ϊ��8��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������һ����Ҫ�Ļ���ԭ�ϣ������Ʊ�һϵ������(����ͼ��ʾ)������˵���������(����)

A. ��ʽ������ˮ���ܲ���Fe(OH)3���壬��������ˮ��
B. Ϊ��ֹNH4HCO3�ֽ⣬����FeCO3���ڽϵ��¶��½���
C. ����KSCN��Һ����(NH4)2Fe(SO4)2�Ƿ�����
D. �����£�(NH4)2Fe(SO4)2��ˮ�е��ܽ�ȱ�FeSO4�Ĵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������0.10 mol��L-1����ֱ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.10 mol��L-1 CH3COONa��Һ��NaCN��Һ�����õζ���������ͼ����������仯��������˵����ȷ����

A. ��Һ�������ӵ����ʵ���Ũ��֮�ͣ���ڵ��ڵ��
B. �����ʾ��Һ�У�c(CN��)+ c(HCN)=2c(Cl��)
C. �����ʾ��Һ�У�c(Na+)> c(Cl-)> c(CH3COO-)> c(CH3COOH)
D. �����ʾ��Һ�У�c(Na+)+ c(CH3COOH)+ c(H+)>0.10 mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ӹ�ҵ����30%��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣
��1��д��FeCl3��Һ�����ͭ������Ӧ�����ӷ���ʽ��_________________________��
��2��ij����ʦΪ�˴�ʹ�ù��ĸ�ʴ��Һ�л���ͭ�������»�ô�����FeCl3��Һ��������ͼ��ʾ���裺
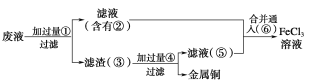
A��д������ʵ���м�������ɵ��й����ʵĻ�ѧʽ��
��________����________����________����________����________����________��
B��д�����й����е����ӷ���ʽ��
�١���___________________________________��
�ݣ���_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ�ÿ�������ȡ�۲�Fe(OH)2�ڿ����б�����ʱ��ɫ�ı仯��ʵ��ʱ����ʹ����м��6mol��L1�����ᣬ�����Լ���ѡ����д���пհף�
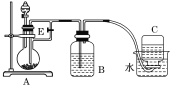
��1��B��ʢһ������NaOH��Һ��A��ӦԤ�ȼ�����Լ���________��A�з�Ӧ�����ӷ���ʽ��________________________________________��
��2��ʵ�鿪ʼʱ���Ƚ�����E________(����رա�)��C���ռ����������Ҫ�ɷ���________��
��3����������Fe(OH)2�IJ�������________________________________________��
��4����ȥװ��B�е���Ƥ����ʹ�������룬װ��B�з���������Ϊ ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����η�ֹ������ʴ�ǹ�ҵ���о����ص����ݡ�Ϊ�о�������ʴ,ijͬѧ����̽��ʵ��,����ͼ��ʾ,�����ڢ٢ڢ����ֲ�ͬ�Ļ�����

��ش�:
��1��������ʴ��Ҫ��Ϊ__________��ʴ��_____________��ʴ���֡�
��2��������ʴ�������ɿ쵽����˳����_________________(�����)��
��3�����������ĵ缫��ӦʽΪ_________________________________________________�����и����ĵ缫��ӦʽΪ_________________________________________��
��4����������ʾ,ȫ����ÿ����ʴ�����ϵĽ��������൱�����������20%���ϡ�Ϊ������������ʴ�ɲ�ȡ�Ĵ�ʩ��__________(�����)��
�ٽ�������ˢ���� �����г���Ȧ�Ƹ�
�۽��ֹ��õ�����ͭ������ �ܽ��ֹ��õ�����̼������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е����λ������ͼ��ʾ������Wԭ�ӵ���������������������������������˵������ȷ������ ��

A. ԭ�Ӱ뾶��W>Z>Y>X
B. ����������Ӧˮ�����������X>W>Z
C. �����̬�⻯������ȶ��ԣ�Y>X>W>Z
D. Ԫ��X��Z��W������ϼ۷ֱ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л����˵������ȷ����( )
A. ��ȥ���������л��е����ᣬ�����ñ���̼������Һ
B. ˳-2-��ϩ�뷴-2-��ϩ�������ӳɵIJ��ﲻ��ͬ
C. �ۺ���![]() ���ɵ���CH3CH��CH2��CH2��CH2�Ӿ��Ƶ�
���ɵ���CH3CH��CH2��CH2��CH2�Ӿ��Ƶ�
D. C3H2Cl6��4��ͬ���칹��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������{[CH3CH(OH)COO]2Fe��3H2O}(��Է���������288)����������FeCO3��Ӧ�Ƶã���������ˮ����һ�ֺܺõIJ�������
I���Ʊ�̼��������װ����ͼ��ʾ��

(1)����B��������____________��
(2)ʵ��������£��رջ���2������1��3����������ϡ���ᷴӦһ��ʱ�䣬��Ŀ���ǣ�_________��Ȼ��رջ���1���������IJ����ǣ�_________________��C�з�����Ӧ�����ӷ���ʽΪ_______________________________��
���Ʊ�����������
����FeCO3�����������������Һ����75���½���ʹ֮��ַ�Ӧ��
(3)�÷�Ӧ����ʽΪ_______________________________________��Ϊ��ֹ�����������ʣ���������ϵ�л�Ӧ����______________________����Ӧ������������Һ��������������������ȴ�ᾧ�����ˡ�ϴ�ӡ�����������������塣�þ�����ʱӦע��_______��
III�������������崿�ȵIJ�����
(4)��λͬѧ�ֱ��ò�ͬ�������вⶨ��
�ټ�ͬѧͨ��KMnO4�ζ����ⶨ��Ʒ��Fe2+�ĺ���������Ʒ���ȣ����ô������Ǵ���100������ԭ�������________________________________��
����ͬѧ�������������(NH4)4Ce(SO4)4�ζ����ⶨ��Ʒ��Fe2+�ĺ���������Ʒ����(��Ӧ��Ce4+��ԭΪCe3+)����ȡ6.00g��Ʒ���Ƴ�250.00mL��Һ��ȡ25.00mL��0.10mol��L-1(NH4)4Ce(SO4)4����Һ�ζ����յ㣬���ı�Һ20.00mL�����Ʒ��������������Ĵ���Ϊ__________(������������ʾ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com