
”¾ĢāÄæ”æŅ»¶ØĢõ¼žĻĀ£¬Fe”¢HCNÓėK2CO3æÉ·¢Éś·“Ó¦Fe+6HCN+2K2CO3=K4Fe(CN)6+H2”ü+2CO2”ü+2H2O”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)½šŹōĢśµÄ¶Ń»ż·½Ź½ĪŖ___________£¬ĘäÅäĪ»ŹżĪŖ___________”£
(2)HCN·Ö×ӵĽį¹¹Ź½ĪŖ___________£¬Š“³öŅ»ÖÖÓėCN£»„ĪŖµČµē×ÓĢåµÄŅõĄė×Ó£ŗ___________”£
(3)¼ü½ĒNH3___________(Ģī”°>”±”°<”±»ņ”°=")NF3£¬ŌŅņŹĒ___________”£
(4)K4Fe(CN)6µÄÖŠŠÄĄė×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ___________”£
(5)C”¢N”¢O”¢HµÄµŚŅ»µēĄėÄÜÓÉŠ”µ½“óµÄĖ³ŠņĪŖ___________”£
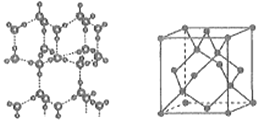
(6)±łµÄ¾§Ģå½į¹¹Ä£ŠĶČēĶ¼ĖłŹ¾£¬Ę侧°ū½į¹¹(ČēĶ¼ĖłŹ¾)Óė½šøÕŹÆµÄ¾§°ū½į¹¹ĻąĖĘ£¬Ė®·Ö×Ó¼äŅŌĒā¼üĻąĮ¬£¬ŌņŅ»øö¾§°ūÖŠŗ¬ÓŠ___________øöĒā¼ü£¬ÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ£¬ČōĒā¼üµÄ¼ü³¤ĪŖdnm£¬Ōņ¾§ĢåĆܶȦŃ=___________g”¤cm£3(ÓĆŗ¬ÓŠd”¢NAµÄ“śŹżŹ½±ķŹ¾)”£
”¾“š°ø”æĢåŠÄĮ¢·½¶Ń»ż 8 ![]() C22- £¾ N¾ł²ÉÓĆsp3Ōӻƣ¬¾łÓŠŅ»¶Ō¹Āµē×Ó¶Ō£¬¶ųµēøŗŠŌF£¾N£¬¹ŹNF3ÖŠ³É¼üµē×Ó¶ŌŌ¶ĄėÖŠŠÄŌ×ÓN£¬ÅųāĮ¦½ĻŠ”£¬¼ü½Ē½ĻŠ” 1s22s22p63s23p63d6»ņ[Ar]3d6 C£¼H£¼O£¼N 16
C22- £¾ N¾ł²ÉÓĆsp3Ōӻƣ¬¾łÓŠŅ»¶Ō¹Āµē×Ó¶Ō£¬¶ųµēøŗŠŌF£¾N£¬¹ŹNF3ÖŠ³É¼üµē×Ó¶ŌŌ¶ĄėÖŠŠÄŌ×ÓN£¬ÅųāĮ¦½ĻŠ”£¬¼ü½Ē½ĻŠ” 1s22s22p63s23p63d6»ņ[Ar]3d6 C£¼H£¼O£¼N 16 ![]()
”¾½āĪö”æ
£Ø1£©½šŹōĢśµÄ½ōĆܶѻż·½Ź½ĪŖĢåŠÄĮ¢·½¶Ń»ż£¬ĢåŠÄFeŌ×ÓÓė¶„µć8øöFeŌ×ÓĻąĮŚ£¬ĘäÅäĪ»ŹżĪŖ8”£
£Ø2£©HCNĢ¼ŠĪ³É2øö¦Ä¼ü£¬2øö¦Š¼ü£¬HCN·Ö×ӵĽį¹¹Ź½ĪŖ![]() £¬ÓėCN-»„ĪŖµČµē×ÓĢåµÄŅõĄė×ÓŹĒC22-”£
£¬ÓėCN-»„ĪŖµČµē×ÓĢåµÄŅõĄė×ÓŹĒC22-”£
£Ø3£©ÓÉÓŚN¾ł²ÉÓĆsp3Ōӻƣ¬¾łÓŠŅ»¶Ō¹Āµē×Ó¶Ō£¬¶ųµēøŗŠŌF£¾N£¬¹ŹNF3ÖŠ³É¼üµē×Ó¶ŌŌ¶ĄėÖŠŠÄŌ×ÓN£¬ÅųāĮ¦½ĻŠ”£¬¼ü½Ē½ĻŠ”£¬ĖłŅŌNH3ŗĶNF3¼ü½Ē²»Ķ¬”£
£Ø4£©ÅäŗĻĪļK4Fe(CN)6µÄÖŠŠÄĄė×ӵĵē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d6»ņ[Ar]3d6”£
£Ø5£©C”¢N”¢O“¦ÓŚĶ¬Ņ»ÖÜĘŚŌŖĖŲÖŠ£¬ŌŖĖŲµÄµŚŅ»µēĄėÄÜĖę×ÅŌ×ÓŠņŹżµÄŌö“ó¶ų³ŹŌö“óĒ÷ŹĘ£¬µ«µŚ¢ņA×壬µŚ¢õA×åŌŖĖŲµÄµŚŅ»µēĄėÄÜ“óÓŚĻąĮŚŌŖĖŲ£¬ĖłŅŌĖüĆĒµÄµēĄėÄÜÓÉŠ”µ½“óµÄĖ³ŠņŹĒC<O<N£¬Ķ¬Ź±H²»ŌŚµŚ¶žÖÜĘŚ£¬Ņ²²»ÓėC”¢N”¢OĶ¬Ö÷×壬²»ÄÜÖ±½ÓÅŠ¶Ļ£¬æɲĪæ¼µć¾¦ÖŠĶ¼Ļń£¬HŌŚCŗĶOÖ®¼ä£¬ĖłŅŌ×īÖÕ“š°øC<H<O<N”£
£Ø6£©Ņ»øö¾§°ūÖŠĖ®·Ö×ÓŹżĪŖ8”Į1/8+6”Į1/2+4=8øö£¬¾§Ģå±łÖŠ£¬ĆæĮ½øöĖ®·Ö×Ó¼äÓŠŅ»øöĒā¼ü£¬Ę½¾łŹōÓŚĆæøöĖ®·Ö×ÓÓŠ°ėøö£¬Ņ»øöĖ®·Ö×ÓÓėÖÜĪ§µÄĖÄøöĖ®·Ö×ÓŅŌĒā¼ü½įŗĻ£¬¹Ź1mol±łÖŠÓŠ2molĒā¼ü£¬ŌŚŅ»øö¾§°ūÖŠÓŠ8”Į2=16øöĒā¼ü£¬ČōĒā¼ü¼ü³¤ĪŖdnm£¬Ōņ¾§ĢåĆܶČ(g”¤cm-3)¼ĘĖćŹ½ĪŖ![]() ”£
ӣ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æōĒ»ł×ŌÓÉ»ł(”¤OH£¬µēÖŠŠŌ£¬OĪŖ-1¼Ū)ŹĒŅ»ÖÖ»īŠŌŗ¬ŃõĪ¢Į£”£³£ĪĀĻĀ£¬ĄūÓĆ”¤OH“¦Ąķŗ¬±½·Ó·ĻĖ®£¬æɽ«Ęä×Ŗ»ÆĪŖĪŽ¶¾µÄŃõ»ÆĪļ”£
(1)”¤OHµÄµē×ÓŹ½ĪŖ________”£
(2) pH=3Ź±Fe2+“ß»ÆH2O2µÄ·Ö½ā¹ż³ĢÖŠ²śÉś”¤OHÖŠ¼äĢ壬“ß»ÆŃ»··“Ó¦ČēĻĀ”£½«ii²¹³äĶźÕū”£
i.Fe2++ H2O2+H+ === Fe3++ H2O +”¤OH
ii.___ + ___ === ___ + O2ӟ+2H+
(3)ŅŃÖŖ£ŗōĒ»ł×ŌÓÉ»łČŻŅ×·¢Éśā§Ćš2”¤OH === H2O2”£ÓĆH2O2·Ö½ā²śÉśµÄ”¤OHĶŃ³ż±½·Ó£¬µ±ĘäĖūĢõ¼ž²»±äŹ±£¬²»Ķ¬ĪĀ¶ČĻĀ£¬±½·ÓµÄÅضČĖꏱ¼äµÄ±ä»ÆČēĻĀĶ¼ĖłŹ¾”£0~20 minŹ±£¬ĪĀ¶Č“Ó40”ęÉĻÉżµ½50”ę£¬·“Ó¦ĖŁĀŹ»ł±¾²»±äµÄŌŅņŹĒ________”£


(4)ĄūÓƵē»Æѧøß¼¶Ńõ»Æ¼¼ŹõæÉŅŌŌŚµē½ā²ŪÖŠ³ÖŠų²śÉś”¤OH£¬Ź¹“¦Ąķŗ¬±½·Ó·ĻĖ®øü¼Óøߊ§£¬×°ÖĆČēÉĻĶ¼ĖłŹ¾”£ŅŃÖŖa¼«Ö÷ŅŖ·¢ÉśµÄ·“Ó¦ŹĒO2Éś³ÉH2O2£¬Č»ŗóŌŚµē½āŅŗÖŠ²śÉś”¤OH²¢ŃøĖŁÓė±½·Ó·“Ó¦”£
¢Ł b¼«Į¬½ÓµēŌ“µÄ________¼«(Ģī”°Õż”±»ņ”°øŗ”±)”£
¢Śa¼«µÄµē¼«·“Ó¦Ź½ĪŖ________”£
¢Ūµē½āŅŗÖŠ·¢ÉśµÄÖ÷ŅŖ·“Ó¦·½³ĢŹ½ĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”湤ŅµÉś²śÖŠ£¬ĻņNa2CO3ČÜŅŗÖŠĶØČėSO2ĘųĢåÖʱøĪŽĖ®Na2SO3£¬Ė®ČÜŅŗÖŠH2SO3”¢HSO3-”¢SO32-Į£×ÓµÄĪļÖŹµÄĮæ·ÖŹżĖępHµÄ·Ö²¼”¢Na2SO3µÄČܽā¶ČĒśĻßČēĶ¼ĖłŹ¾£ŗ

ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£ŗ
A. ČÜŅŗpH=4Ź±£¬ČÜÖŹĪŖNaHSO3
B. ČÜŅŗpH=10Ź±£¬c(Na+)+ c(H+)= c(OHØC)+ c(SO32ØC)+ c(HSO3ØC)
C. ČÜŅŗpH=7Ź±£¬ c(SO32ØC)= c(HSO3ØC)
D. ČÜŅŗpH=10Ź±£¬Ķ£Ö¹ĶØČėSO2£¬½«ČÜŅŗ¼ÓČČÅØĖõÖĮÓŠ“óĮ澧ĢåĪö³ö£¬ŌŚøßÓŚ34”ę³ĆČČ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµĆµ½ĪŽĖ®Na2SO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ³£ĪĀĻĀ£¬½«20.0g14.0%µÄNaClČÜŅŗøś30.0g24.0%µÄNaClČÜŅŗ»ģŗĻ£¬µĆµ½ĆܶČĪŖ1.15g/cm3µÄ»ģŗĻČÜŅŗ”£Ēó£ŗ
(1)øĆ»ģŗĻČÜŅŗµÄÖŹĮæ·ÖŹżĪŖ_________£æ
(2)øĆ»ģŗĻČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ_________£æ
(3)ŌŚ1000gĖ®ÖŠČܽā_______Ħ¶ūNaCl²ÅÄÜŹ¹ĘäÅضČÓėÉĻŹöČÜŅŗµÄÅضČĻąµČ?
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æķŚ(Te)¾ßÓŠĮ½ŠŌĢŲÕ÷£¬ĶŃō¼«ÄąŹĒĢįČ”ķŚµÄÖ÷ŅŖŌĮĻ£¬ķŚŌŚĶŃō¼«ÄąÖŠÖ÷ŅŖŅŌMe2Te(Me±ķŹ¾½šŹōCu”¢Pb”¢Au”¢AgµČ)µÄŠĪŹ½“ęŌŚ”£
(1)ĄūÓĆ”°ĀČ»Æ½ž³ö-»¹Ō·Ø”±ÖʱøTeµÄ¹¤ŅÕĮ÷³ĢČēĻĀĖłŹ¾”£

¢Ł½ž³öŅŗµÄÖ÷ŅŖ³É·ÖĪŖCuSO4”¢ HAuCl4”¢H2TeO3£¬Ōņ½ž³öŌüµÄÖ÷ŅŖ³É·ÖĪŖ___________(Ģī»ÆѧŹ½)£»”°½ž³ö”±¹ż³ĢÖŠ£¬¼ÓČėNaClµÄ×÷ÓĆŹĒ___________”£”°½ž³ö”±¹ż³ĢÖŠ£¬ÓŠÉŁĮæĪŪČ¾ŠŌĘųĢåÉś³É£¬ŠčŅŖ½ųŠŠĪ²Ęų“¦Ąķ£¬øĆĘųĢåŹĒ___________(Ģī»ÆѧĆū³Ę)
¢ŚŅŃÖŖ HAuCl4ŹĒŅ»ÖÖĒæĖį£¬Ōņ”°Ņ»¼¶»¹Ō”±¹ż³ĢÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ___________”£
¢ŪÓūµĆµ½64gķŚ£¬Ōņ”°¶ž¼¶»¹Ō”±¹ż³ĢÖŠÖĮÉŁŠčĶØČė___________mol SO2”£
(2)”°Ńõ»Æ¼ī½ž-µē½ā·Ø”±ÖøµÄŹĒŌŚŃõ»Æ¼ĮµÄ×÷ÓĆĻĀ£¬Cu2TeÓėNaOHČÜŅŗ·“Ӧɜ³ÉNa2TeO3£¬¾µē½ā¼“æÉ»ńµĆTe”£
¢ŁŅŌæÕĘųĪŖŃõ»Æ¼Į½ųŠŠ”°Ńõ»Æ¼ī½ž”±µÄ»Æѧ·½³ĢŹ½ĪŖ___________”£
¢Śµē½ā¹ż³ĢÖŠ£¬Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖ___________”£
¢ŪÓė”°ĀČ»Æ½ž³ö-»¹Ō·Ø”±Ļą±Č”°Ńõ»Æ¼ī½ž-µē½ā·Ø”±µÄÓŵćŹĒ___________(ČĪŠ“Ņ»µć)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓĆCl2Éś²śŗ¬ĀČÓŠ»śĪļŹ±»į²śÉśHCl£¬ĄūÓĆČēĻĀ·“Ó¦æÉŹµĻÖĀȵÄŃ»·ĄūÓĆ”£4HCl(g)+O2(g)=2Cl2(g)+2H2O(g)+Q(Q>0)ŌŚ2LĆܱÕČŻĘ÷ÖŠ½ųŠŠøĆ·“Ó¦£¬ŌŚ²»Ķ¬Ź±¼ä²āµĆŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ
ĪļÖŹ ĪļÖŹµÄĮæ (mol) Ź±¼ä | HCl(g) | O2(g) | H2O(g) | Cl2(g) |
0min | 4 | 1 | 0 | 0 |
2min | 1.2 | 0.3 | 1.4 | |
3min | 1.2 | 0.3 | 1.4 | |
4min | 1.0 | 0.35 | 1.5 |
(1)¼ĘĖć0~2minÄŚCl2µÄĘ½¾łÉś³ÉĖŁĀŹ___________________”£ øĆ·“Ó¦ŗĻŹŹµÄĪĀ¶Č·¶Ī§ŹĒ380~440”ę£¬Ń”ŌńøĆĪĀ¶Č·¶Ī§æÉÄܵÄŌŅņŹĒ£ŗ¢Ł¼Óæģ·“Ó¦ĖŁĀŹ£»¢Ś_______________________________”£
(2)¹¤ŅµÉĻÓĆ“«øŠĘ÷¼ģ²ā·“ӦȯĘ÷ÖŠµÄĘųĢåŃ¹Ē棬µ±Ń¹Ēæ±£³Ö²»±äŹ±£¬±ķĆę·“Ó¦ŅŃ“ļĘ½ŗā”£ÄÜĶعż¼ģ²āŃ¹ĒæÅŠ¶Ļ·“Ó¦ŹĒ·ń“ļµ½Ę½ŗāדĢ¬µÄĄķÓÉŹĒ__________________________”£
(3)ŹµŃ鏱£¬ŌŚ3~4minÖ®¼äøıäĮĖijŅ»Ģõ¼žŌģ³ÉĘ½ŗāŅĘ¶Æ”£±ķÖŠŹż¾ŻĖµĆ÷Ę½ŗāĻņ____ŅʶÆ(Ģī”°×ó”±»ņ”°ÓŅ”±)£»“ļµ½ŠĀĘ½ŗāŗó£¬ÓėŌĄ“Ļą±ČĘ½ŗā³£Źż_______(Ģī”°±ä“ó”±”¢”°±äŠ””±”¢”°²»±ä”±)”£
(4)Cl2Ņ²æÉÓĆÓŚÖʱøŠĀŠĶ¾»Ė®¼ĮøßĢśĖįÄĘ(Na2FeO4)”£ÅäĘ½Öʱø·“Ó¦µÄ»Æѧ·½³ĢŹ½___Fe(NO3)3+___NaOH+___Cl2”ś___Na2FeO4+___NaNO3+___NaCl+___H2OČō·“Ó¦ĻūŗÄ3.36L Cl2(±ź×¼×“æö)£¬Ōņ×ŖŅʵē×ӵďżÄæŹĒ________”£
(5)ÓĆNa2FeO4ɱ¾śĻū¶¾µÄÓŵćŹĒ»¹Ō²śĪļ¾ßÓŠ¾»Ė®×÷ÓĆ£¬Č±µćŹĒ»¹Ō²śĪļ»į¶ŌĢśÖĘĖ®¹ÜŌģ³ÉøÆŹ“£¬Ōģ³ÉøÆŹ“µÄŌĄķÓŠ________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖ£ŗŅŅ¶žĖįĖ×³Ę²ŻĖį(½į¹¹¼ņŹ½ĪŖHOOCCOOH£¬æɼņŠ“ĪŖH2C2O4)”£25”ꏱ£¬²ŻĖįøʵÄKsp=4.0”Į108£¬Ģ¼ĖįøʵÄKsp=2.5”Į109”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)25”ꏱĻņ20mLĢ¼Ėįøʵı„ŗĶČÜŅŗÖŠÖšµĪ¼ÓČė8.0”Į104mol/LµÄ²ŻĖį¼ŲČÜŅŗ20mL£¬ÄÜ·ń²śÉś³Įµķ________(Ģī”°ÄÜ”±»ņ”°²»ÄÜ”±)”£
(2)ĖįŠŌKMnO4ČÜŅŗÄÜÓė²ŻĖį(H2C2O4)ČÜŅŗ·“Ó¦£¬Ä³Ģ½¾æŠ”×éĄūÓĆ·“Ó¦¹ż³ĢÖŠČÜŅŗ×ĻÉ«ĻūŹ§æģĀżµÄ·½·ØĄ“ŃŠ¾æÓ°Ļģ·“Ó¦ĖŁĀŹµÄŅņĖŲ”£
I£®ŹµŃéĒ°Ź×ĻČÓĆÅضČĪŖ0.1000molL1ĖįŠŌKMnO4±ź×¼ČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄ²ŻĖį”£
¢ŁµĪ¶Ø¹ż³ĢÖŠ²Ł×÷µĪ¶Ø¹ÜµÄĶ¼Ź¾ÕżČ·µÄŹĒ________(Ģī±ąŗÅ)”£

¢ŚÅŠ¶ĻµĪ¶ØÖÕµćµÄ·½·ØŹĒ£ŗ________”£
¢ŪČōÅäÖĘĖįŠŌKMnO4±ź×¼ČÜŅŗŹ±£¬ø©ŹÓČŻĮæĘæµÄæĢ¶ČĻߣ¬»įŹ¹²āµĆµÄ²ŻĖįČÜŅŗÅضČ__________(Ģī”°Ę«øß”±»ņ”°Ę«µĶ”±»ņ”°²»±ä”±)”£
¢ņ£®ĶعżµĪ¶ØŹµŃéµĆµ½²ŻĖįČÜŅŗµÄÅضČĪŖ0.2000molL1£®ÓĆøĆ²ŻĖįČÜŅŗ°“ĻĀ±ķ½ųŠŠŗóŠųŹµŃé(Ćæ“ĪŹµŃé²ŻĖįČÜŅŗµÄÓĆĮæ¾łĪŖ8mL)”£
ŹµŃ鱹ŗÅ | ĪĀ¶Č (”ę) | “߻ƼĮÓĆĮæ (g) | ĖįŠŌøßĆĢĖį¼ŲČÜŅŗ | ŹµŃéÄæµÄ a£®ŹµŃé1ŗĶ2Ģ½¾æ | |
Ģå»ż(mL) | ÅضČ(molL1) | ||||
1 | 25 | 0.5 | 4 | 0.1000 | |
b£®ŹµŃé1ŗĶ3Ģ½¾æ·“Ó¦ĪļÅØ¶Č¶ŌøĆ·“Ó¦ĖŁĀŹµÄÓ°Ļģ£»c£®ŹµŃé1ŗĶ4Ģ½¾æ“߻ƼĮ¶ŌøĆ·“Ó¦ĖŁĀŹµÄÓ°Ļģ”£ | |||||
2 | 50 | 0.5 | 4 | 0.1000 | |
3 | 25 | 0.5 | 4 | 0.0100 | |
4 | 25 | 0 | 4 | 0.1000 | |
¢ÜŠ“³ö±ķÖŠa¶ŌÓ¦µÄŹµŃéÄæµÄ________£»
¢ŻøĆŠ”×éĶ¬Ń§¶ŌŹµŃé1ŗĶ3·Ö±š½ųŠŠĮĖČż“ĪŹµŃ飬²āµĆŅŌĻĀŹµŃ鏿¾Ż(“Ó»ģŗĻÕńµ“¾łŌČæŖŹ¼¼ĘŹ±)£ŗ
ŹµŃ鱹ŗÅ | ČÜŅŗĶŹÉ«ĖłŠčŹ±¼ä(min) | ||
µŚ1“Ī | µŚ2“Ī | µŚ3“Ī | |
1 | 14.0 | 13.0 | 11.0 |
3 | 6.5 | 6.7 | 6.8 |
·ÖĪöÉĻŹöŹż¾ŻŗóµĆ³ö”°µ±ĘäĖüĢõ¼žĻąĶ¬Ź±£¬ĖįŠŌøßĆĢĖį¼ŲČÜŅŗµÄÅضČŌ½Š”£¬ĶŹÉ«Ź±¼ä¾ĶŌ½¶Ģ£¬¼“·“Ó¦ĖŁĀŹ¾ĶŌ½æģ”±µÄ½įĀŪ”£Ä³Ķ¬Ń§ČĻĪŖøĆŠ”×é”°Ģ½¾æ·“Ó¦ĪļÅØ¶Č¶ŌĖŁĀŹÓ°Ļģ”±µÄŹµŃé·½°øÉč¼ĘÖŠ“ęŌŚĪŹĢā£¬“Ó¶ųµĆµ½ĮĖ“ķĪóµÄŹµŃé½įĀŪ£¬Ēė¼ņŹöøĽųµÄŹµŃé·½°ø______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijĢžµÄ½į¹¹¼ņŹ½£ŗ![]() £¬ĻĀĮŠÓŠ¹ŲĘä¼øŗĪ½į¹¹µÄĖµ·ØÕżČ·µÄŹĒ
£¬ĻĀĮŠÓŠ¹ŲĘä¼øŗĪ½į¹¹µÄĖµ·ØÕżČ·µÄŹĒ
A. ĖłÓŠĢ¼Ō×Ó²»æÉÄÜŌŚĶ¬Ņ»Ę½ĆęÉĻ B. ÓŠ4øöĢ¼Ō×ÓŌŚĶ¬Ņ»Ö±ĻßÉĻ
C. ÓŠ5øöĢ¼Ō×ÓŌŚĶ¬Ņ»Ö±ĻßÉĻ D. ÓŠ6øöĢ¼Ō×ÓŌŚĶ¬Ņ»Ö±ĻßÉĻ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅ»øöĆܱÕČŻĘ÷£¬ÖŠ¼äÓŠŅ»æÉ×ŌÓÉ»¬¶ÆµÄøō°å£Øŗń¶Č²»¼Ę£©½«ČŻĘ÷·Ö³ÉĮ½²æ·Ö£¬µ±×ó±ß³äČė 1mol N2£¬ ÓŅ±ß³äČė CO ŗĶ CO2 µÄ»ģŗĻĘųĢå¹² 8g Ź±£¬øō°å“¦ÓŚČēĶ¼Ī»ÖĆ£Ø±£³ÖĪĀ¶Č²»±ä£©£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ

A. ÓŅ±ß CO Óė CO2 ·Ö×ÓŹżÖ®±ČĪŖ 1:3
B. ÓŅ²ą CO µÄÖŹĮæĪŖ 2.75g
C. ČōøıäÓŅ±ß CO ŗĶ CO2 µÄ³äČėĮæ¶ųŹ¹øō°å“¦ÓŚĄėÓŅ¶Ė1/6“¦£¬ ±£³ÖĪĀ¶Č²»±ä£¬ŌņĒ°ŗóĮ½“Ī³äČėČŻĘ÷ÄŚµÄĘųĢåŃ¹ĒæÖ®±ČĪŖ 5: 3
D. ÓŅ²ąĘųĢåĆܶȏĒĻąĶ¬Ģõ¼žĻĀĒāĘųĆÜ¶ČµÄ 16 ±¶
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com