
 ��Һ
��Һ �õ�����������Ϊm2g��
�õ�����������Ϊm2g�� ���ɵ������ڱ�״���µ����ΪV1L��
���ɵ������ڱ�״���µ����ΪV1L�� ���ɵ������ڱ�״���µ����ΪV2L��
���ɵ������ڱ�״���µ����ΪV2L��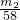 mol������þԭ���غ㣬�Ͻ��н���þ����������Ϊ��
mol������þԭ���غ㣬�Ͻ��н���þ����������Ϊ�� ��100%=
��100%=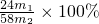 ��
��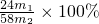 ��
��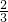 n��H2��=
n��H2��=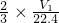 mol���Ͻ��н���������������Ϊ��
mol���Ͻ��н���������������Ϊ��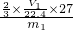 ��100%=
��100%= ��100%���Ͻ����������������ǣ�1-
��100%���Ͻ����������������ǣ�1- ��100%=
��100%= ��
�� ��
��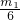 -
-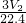 ������þ���Ͻ���þ�����������ǣ�
������þ���Ͻ���þ�����������ǣ�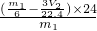 ��100%=
��100%=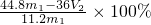 ��
��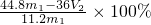 ��
��

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���������� |
| �ڹ���NaOH��Һ |
| ���� |
| ������NaOH��Һ |
| �������� |
| 24m1 |
| 58m2 |
| 24m1 |
| 58m2 |
| 9V1 |
| 11.2m1 |
| 9V1 |
| 11.2m1 |
| 44.8m1-36V2 |
| 11.2m1 |
| 44.8m1-36V2 |
| 11.2m1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̽��һ��
ʵ�鷽������þ�Ͻ�![]() �ⶨʣ���������
�ⶨʣ���������
ʵ���з�����Ӧ�Ļ�ѧ����ʽ��______________________��
ʵ�鲽�裺��1����ȡ
��2�����ˡ�ϴ�ӡ�����������塣�ò�������δϴ�ӹ��壬���þ������������_________���ƫ�ߡ���ƫ�͡�����
��̽������
ʵ�鷽������þ�Ͻ�![]() �ⶨ������������
�ⶨ������������
ʵ��װ�ã�

�������ۣ���1��ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ�������������װ�á���������_____________�������Ҫ������Ҫ����
��2��Ϊʹ�ⶨ��������ܾ�ȷ��ʵ����Ӧע��������ǣ�д�����㣩��
��_______________________________,��________________________________��
��̽������
ʵ�鷽��������x g��þ�Ͻ��ĩ����������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������ա�

�������ۣ���1��������Mg��������������ʵ���л���ⶨ��������____________��
��2�����ÿ�������O2����ʵ�飬�Բⶨ����Ƿ���Ӱ�죿___________����ǡ�����
��ʵ����չ��
����̽��һ��̽������ʵ�鷽�������������һ��ʵ�鷽�����ⶨ����þ�Ͻ���þ������������
____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ��ɽ��ʡ�����и�һ���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
 ��Һ
��Һ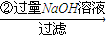 �õ�����������Ϊm2g��
�õ�����������Ϊm2g�� ���ɵ������ڱ�״���µ����ΪV1L��
���ɵ������ڱ�״���µ����ΪV1L�� ���ɵ������ڱ�״���µ����ΪV2L��
���ɵ������ڱ�״���µ����ΪV2L���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com