
��Ϣʱ�������Ĵ������������Ի��������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70% Cu��25% Al��4% Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�
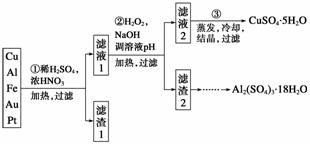
��ش��������⣺
(1)�ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ_______________________________________
________________________________________________________________________��
�õ�����1����Ҫ�ɷ�Ϊ____________��
(2)�ڢڲ���H2O2��������______________��ʹ��H2O2���ŵ���______________������ҺpH��Ŀ����ʹ______________���ɳ�����
(3)�õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ�����________________________��
(4)������2��ȡAl2(SO4)3��18H2O ��̽��С����������ַ�����
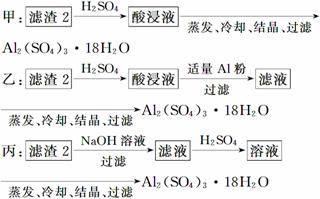
�������ַ����У�________���������У�ԭ����_______________________________
________________________________________________________________________��
��ԭ�������ʽǶȿ��ǣ�__________������������
�𰸡�(1)Cu��4H����2NO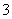
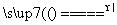 Cu2����2NO2����2H2O
Cu2����2NO2����2H2O
��3Cu��8H����2NO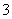
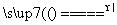 3Cu2����2NO����4H2O��Au��Pt
3Cu2����2NO����4H2O��Au��Pt
(2)��Fe2������ΪFe3�������������ʣ��Ի�������Ⱦ��Fe3����Al3��
(3)������ˮ
(4)�ס����ò�Ʒ�к��н϶�Fe2(SO4)3���ʡ���
�����������Ʊ�·�߿�֪��Cu��Al��Fe��Au��Pt�Ļ�����м���ϡH2SO4��Ũ���ᣬ����Խ�Cu��Al��Fe�ܽ⣬Au��Pt���ܽ⣬��������1����Ҫ�ɷ�ΪAu��Pt����Һ1�к���Cu��Fe��Al�����ӡ�������Һ2��CuSO4��5H2O��֪����Һ2ΪCuSO4��Һ������2�к���Fe(OH)3��Al(OH)3��
(1)�ڢٲ���Cu���ᷢ���ķ�ӦΪCu��Ũ����ķ�Ӧ��Cu��4H����2NO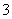
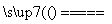 Cu2����2NO2����2H2O��Ũ������ϡ����Ļ�Ϲ����������ϡ�������ķ�ӦҲ����Ϊ3Cu��8H����2NO
Cu2����2NO2����2H2O��Ũ������ϡ����Ļ�Ϲ����������ϡ�������ķ�ӦҲ����Ϊ3Cu��8H����2NO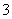
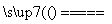 3Cu2����2NO����4H2O�����ݷ�����֪����1����Ҫ�ɷ�ΪPt��Au��
3Cu2����2NO����4H2O�����ݷ�����֪����1����Ҫ�ɷ�ΪPt��Au��
(2)�ڢڲ������м�H2O2��Ŀ���ǽ�Fe2��ת��ΪFe3�����Ӷ���Fe3��ת��Ϊ������ȥ����ֹ��CuSO4������Ʊ��������š�H2O2���ŵ��ǻ�ԭ����ΪH2O�����������ʣ�ͬʱ�Ի���û����Ⱦ������pH��Ŀ���ǽ�Fe3����Al3��ת��Ϊ��������ȥ��
(3)��CuSO4��5H2O�Ʊ���ˮCuSO4��ֻҪ��ȥ�ᾧˮ���ɡ��ڼ��ȹ�����CuSO4����ˮ�⣬������Ϊ����ӷ����������յõ�����Ȼ��CuSO4������ֻҪ������ˮ���ɡ�
(4)�����Ʊ���Al2(SO4)3�����к���Fe2(SO4)3���ҡ������ַ����У��ҷ�����ԭ�������ʸ��ߡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���ķ���ʽΪC9H9ClO2 �������к���1��������������������ȡ������������NaHCO3��Һ��Ӧ��ͬ���칹�����ĿΪ���������������ͣ�
A��12�� B�� 15�� C��18�� D. 21��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵķ����У�������������ṹ���� �� ��
A��CHCl3 B��CCl4 C��CH2Cl2 D��CH3Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����о�С�飬�ú��н϶����ʵ�ͭ�ۣ�ͨ����ͬ�Ļ�ѧ��Ӧ����CuO������Ƶ�ʵ�����Ϊ

(1)��ͭ�����պ�õ��IJ���������ͭ������ͭ�Ļ������պ�������ͭ�Ŀ���ԭ����________(����ĸ���)��
a�����չ����в�������ͭ����ԭ
b�����ղ����ͭδ����ȫ����
c������ͭ�ڼ��ȹ����зֽ�����ͭ
d����������ͭ������������
(2)�ɴ�������ͭͨ������;����ȡ����CuO����;������ȣ�;���������Ե������ŵ���______________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���н���ұ���ķ�Ӧԭ����������� (����)
A��MgCl2(����)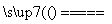 Mg��Cl2��
Mg��Cl2��
B��Al2O3��3H2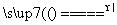 2Al��3H2O
2Al��3H2O
C��Fe3O4��4CO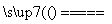 3Fe��4CO2
3Fe��4CO2
D��2HgO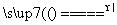 2Hg��O2��
2Hg��O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�⻯��ͭ(CuH)��һ�������ʣ���CuSO4��Һ����һ�֡���Ӧ���40��50 ��ʱ��Ӧ����������CuH���ȶ����ֽ⣻CuH����������ȼ�գ������ᷴӦ�ܲ������塣�����й��ƶ��д������ (����)
A������ġ���һ�ַ�Ӧ����л�ԭ��
B��CuH��������������ԭ��
C��CuH��Cl2===CuCl��HCl(ȼ��)
D��CuH��HCl===CuCl��H2��(����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�Ͻ���м����Ҫ�ɷ�ΪCu��Fe��Al��������������ͭ��[Cu2(OH)2CO3]����������������������������������Ͻ���м��ȡ����(CuSO4��5H2O)����ˮAlCl3������Ĺ�������ͼ��ʾ��
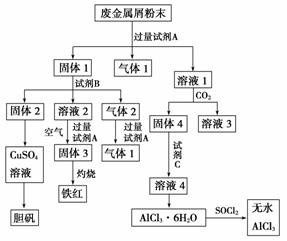
��ش�
(1)�ڷϽ���м��ĩ�м����Լ�A����������1�ķ�Ӧ�����ӷ���ʽ��________________________________________________________________________
________________________________________________________________________��
(2)��Һ2�к��еĽ�����������__________������2�ijɷ���__________��
(3)��Һ2ת��Ϊ����3�ķ�Ӧ�����ӷ���ʽ��________________________________
________________________________________________________________________��
(4)���ù���2��ȡCuSO4��Һ�ж��ַ�����
���ڹ���2�м���ŨH2SO4�����ȣ�ʹ����2ȫ���ܽ��CuSO4��Һ����Ӧ�Ļ�ѧ����ʽ��_______________________________________________ _____________________��
_____________________��
���ڹ���2�м���ϡH2SO4��ͨ��O2�����ȣ�ʹ����2ȫ���ܽ��CuSO4��Һ����Ӧ�����ӷ���ʽ��_________________________________________________________
________________________________________________________________________��
(5)��Һ1ת��Ϊ��Һ4�����У�������Һ1��ֱ�Ӽ����Լ�C��������________________________________________________________________________
________________________________________________________________________��
(6)ֱ�Ӽ���AlCl3��6H2O���ܵõ���ˮAlCl3��SOCl2Ϊ��ɫҺ�壬������ˮ��Ӧ����HCl��һ�־���Ư���Ե����塣AlCl3��6H2O��SOCl2��ϼ�����ȡ��ˮAlCl3����Ӧ�Ļ�ѧ����ʽ��______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ѿ�֪����pH��ʾ��Һ��c(H��)�ĸ�������ͬ��Ҳ���Զ���pOH��ʾ��Һ��c(OH��)�ĸ���������pOH����lg c(OH��)���¶�Tʱˮ�����ӻ�������KW��ʾ�����¶��²����ж���Һ�����Ե�������(����)
A.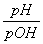 ��1 B��pOH����lg
��1 B��pOH����lg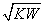
C��c(H��)��10��pOH D��pH��pOH��lg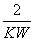
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ����д��ȷ��һ����
�ٹ�����ˮ��FeI2��Һ���ã�
2Fe2+ + 2I- + 2Cl2 == 2 Fe3+ + I2 +4Cl-
�� ������ˮ��Ӧ��2F2��2H2O===4H����4F����O2
�� �����ʯ��ˮ�м�������С�մ���Һ��
Ca2����OH����HCO3��===CaCO3����H2O
�� ͭƬͶ��ϡHNO3��Һ��Cu��NO3����4H��===NO����2H2O��Cu2��
�� Na +2 H2O = Na+ + OH- + H2��
�� ̼��Ʒ��������У� CO32- + 2H+ = CO2�� +H2O
�� ���ˮ�еμ�FeCl3��Һ�Ʊ�Fe(OH)3���壺Fe3+��3H2O Fe(OH)3(����)��3H+
Fe(OH)3(����)��3H+
A. �٢ڢۢ� B. �ۢ� C. �٢ۢܢ� D.�٢ܢޢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com