
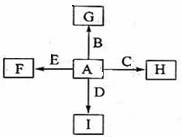
£Ø14·Ö£©ĻĀĶ¼ÖŠ£¬A”¢B”¢C”¢D”¢EŹĒµ„ÖŹ£¬G”¢H”¢I”¢FŹĒB”¢C”¢D”¢E·Ö±šŗĶAŠĪ³ÉµÄ¶žŌŖ»ÆŗĻĪļ”£ŅŃÖŖ£ŗ¢Ł·“Ó¦C+G B+HÄܷųö“óĮæµÄČČ£¬øĆ·“Ó¦ŌųÓ¦ÓĆÓŚĢś¹ģµÄŗø½Ó£»¢ŚIŹĒŅ»ÖÖ³£¼ūµÄĪĀŹŅĘųĢ壬ĖüŗĶEæÉŅŌ·¢Éś·“Ó¦£ŗ2E+I
B+HÄܷųö“óĮæµÄČČ£¬øĆ·“Ó¦ŌųÓ¦ÓĆÓŚĢś¹ģµÄŗø½Ó£»¢ŚIŹĒŅ»ÖÖ³£¼ūµÄĪĀŹŅĘųĢ壬ĖüŗĶEæÉŅŌ·¢Éś·“Ó¦£ŗ2E+I 2F+D£¬FÖŠEŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ60%”£
2F+D£¬FÖŠEŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ60%”£
»Ų“šĪŹĢā£ŗ
¢Å¢ŁÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”””””””””””””””””””””””””””” £»
¢Ę»ÆŗĻĪļIµÄµē×ÓŹ½ĪŖ”””””””””””””” £¬ĖüµÄæÕ¼ä½į¹¹ŹĒ”””””””””””” £»
¢Ē½«GČÜÓŚŃĪĖį£¬µĆµ½µÄČÜŅŗ¼ÓČėŅ»¶ØĮæµÄĶ·Ū£¬Š“³öĄė×Ó·½³ĢŹ½£ŗ
¢ČCÓė¹żĮæNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ””””””””””””””””””””””””””””””””””””””””””£¬·“Ó¦ŗóČÜÓŚÓė¹żĮæ»ÆŗĻĪļI·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ”””””””””””””””””””””””””””””””””””” £»
¢ÉEŌŚIÖŠČ¼ÉÕ¹Ū²ģµ½µÄĻÖĻóŹĒ”””””””””””””””””””””””””””””””””””””””””” ”£
£Ø1£©8Al+3Fe3O4 9Fe+4Al2O3
9Fe+4Al2O3
£Ø2£©
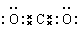 ”” Ö±ĻߊĪ £Ø3£© 2Fe3++Cu=2Fe2++Cu2+
”” Ö±ĻߊĪ £Ø3£© 2Fe3++Cu=2Fe2++Cu2+
£Ø4£©2Al+2OH-+2H2O=2AlO-2+3H2”ü
AlO2-+CO2+2H2O=Al£ØOH£©3”ż+HCO3- £Ø×¢£ŗ²»ŅŖĒóŠ“OH-+CO2=HCO-3£©
£Ø5£©Ć¾Ģõ¾ēĮŅČ¼ÉÕ£¬Éś³É°×É«·ŪÄ©£¬·“Ó¦Ę÷ÄŚ±Śø½ÓŠŗŚÉ«µÄĢ¼
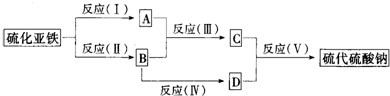
”¾½āĪö”æøł¾Ż¢ŁæÉÖŖ£¬ÕāŹĒĀĮČČ·“Ó¦£¬ĖłŅŌCŹĒĀĮ£¬ŌņHŹĒŃõ»ÆĀĮ£¬AŹĒŃõĘų£¬GŹĒĖÄŃõ»ÆČżĢś£¬B¾ĶŹĒĢś”£³£¼ūµÄĪĀŹŅĘųĢåŹĒCO2£¬ĖłŅŌIŹĒCO2£¬ŌņDŹĒĢ¼”£ÄÜŌŚCO2ÖŠČ¼ÉÕ£¬ÄÜ°ŃCO2ÖŠµÄĢ¼ÖĆ»»³öĄ“µÄŹĒĆ¾£¬¼“EŹĒĆ¾£¬FŹĒŃõ»ÆĆ¾£¬Ńõ»ÆĆ¾ÖŠĆ¾ŌŖĖŲµÄÖŹĮæ·ÖŹżŹĒ60£„”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
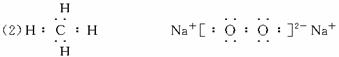
ĻĀĶ¼ÖŠ£¬A”¢B”¢C”¢D”¢E”¢FŌŚ³£ĪĀŹ±¾łĪŖĘųĢ壬A”¢B·Ö×ÓÖŠµÄµē×ÓŹżĻąĶ¬£¬G”¢H¾łĪŖŃõ»ÆĪļ£¬ĘäÖŠ·“Ӧɜ³ÉµÄĖ®¼°ŗ¬Ķ»ÆŗĻĪļ¾łĀŌČ„£¬·“Ó¦Ģõ¼žĪ“×¢Ć÷”£
ŹŌøł¾ŻÉĻĶ¼ø÷ĪļÖŹ¼äµÄ±ä»Æ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³öXµÄ»ÆѧŹ½_________________£»
(2)Š“³öĪļÖŹBŗĶGµÄµē×ÓŹ½£ŗB_________________”¢G_________________£»
(3)·“Ó¦¢ńµÄ»Æѧ·½³ĢŹ½__________________________________£»
(4)·“Ó¦¢ņµÄĄė×Ó·½³ĢŹ½__________________________________£»
(5)ŌŚÉĻŹöø÷²½·“Ó¦ÖŠ£¬ÓŠĮ½ÖÖĪļÖŹ¼Č×÷Ńõ»Æ¼ĮÓÖ×÷»¹Ō¼Į£¬ĖüĆĒŹĒ_________________”¢_________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø8·Ö£©
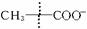
ĻĀĶ¼ÖŠ£¬A”¢B”¢C”¢D”¢EŹĒµ„ÖŹ£¬G”¢H”¢I”¢FŹĒB”¢C”¢D”¢E·Ö±šŗĶAŠĪ³ÉµÄ»ÆŗĻĪļ”£
ŅŃÖŖ£ŗ
¢Ł ·“Ó¦Äܷųö“óĮæµÄČČ£¬øĆ·“Ó¦ŌųÓ¦ÓĆÓŚĢś¹ģµÄŗø½Ó£»
¢Ś IŹĒŅ»ÖÖ³£¼ūµÄĪĀŹŅĘųĢ壬ĖüŗĶ![]() æÉŅŌ·¢Éś·“Ó¦£ŗ2E£«I
æÉŅŌ·¢Éś·“Ó¦£ŗ2E£«I2F£«D£¬FÖŠµÄEŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ60%.
»Ų“šĪŹĢā£ŗ
£Ø1£©¢ŁÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________________________________________£»
£Ø2£©»ÆŗĻĪļ¢ńµÄµē×ÓŹ½ĪŖ______________________£»
£Ø3£©CÓė¹żĮæNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ___________________________;
£Ø4£©ĶĘ²āEŌŚIÖŠČ¼ÉÕæÉÄܹŪ²ģµ½µÄĻÖĻóŹĒ__________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2007ÄźĘÕĶØøßµČѧŠ£ÕŠÉśČ«¹śĶ³Ņ»æ¼ŹŌ£ØČ«¹ś¾ķ¢ń£©Ąķ×Ū»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©””””
ĻĀĶ¼ÖŠµÄA”¢B”¢C”¢D”¢E”¢F”¢G¾łĪŖÓŠ»śĪļ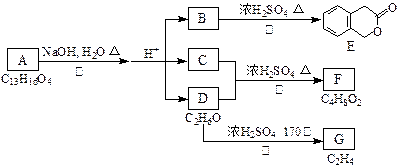
¾ŻÉĻĶ¼»Ų“šĪŹĢā£ŗ
£Ø1£©DµÄ»ÆѧĆū³ĘŹĒ”””””””””£
£Ø2£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ””””””””””””””””””””””””””£ØÓŠ»śĪļŠėÓĆ½į¹¹¼ņŹ½±ķŹ¾£©
£Ø3£©BµÄ·Ö×ÓŹ½ŹĒ£ŗ””””””””””””””””””””””””
AµÄ½į¹¹¼ņŹ½ŹĒ””””””””””””””””·“Ó¦µÄ·“Ó¦ĄąŠĶŹĒ””””””””””””””””””””””””””””
£Ø4£©·ūŗĻĻĀĮŠ3øöĢõ¼žµÄBµÄĶ¬·ÖŅģ¹¹ĢåµÄŹżÄæÓŠ””””øö
¢”£©ŗ¬ÓŠĮŚ¶žČ”“ś±½»·½į¹¹”¢””¢¢£©ÓėBÓŠĻąĶ¬¹ŁÄÜĶÅ”¢¢££©²»ÓėFeCl3ČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦”£
Š“³öĘäÖŠČĪŅāŅ»øöĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½””””””””””””””””””””””””
£Ø5£©GŹĒµÄ¹¤ŅµŌĮĻ£¬ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾GµÄŅ»ÖֵŤŅµÓĆĶ¾””””””””””””””””””””””””
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğČĆɹŰĶŹŠŅ»ÖŠø߶žĻĀѧʌ4ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
ŅŃÖŖĻĀĮŠŹż¾Ż£ŗ
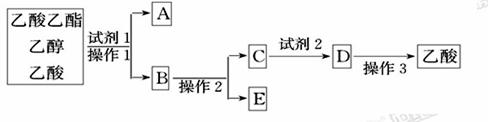
ĻĀĶ¼ĪŖŹµŃéŹŅÖĘČ”ŅŅĖįŅŅõ„µÄ×°ÖĆĶ¼”£
(1)µ±±„ŗĶĢ¼ĖįÄĘČÜŅŗÉĻ·½ŹÕ¼Æµ½½Ļ¶ąŅŗĢåŹ±£¬Ķ£Ö¹¼ÓČČ£¬Č”ĻĀŠ”ŹŌ¹ÜB£¬³ä·ÖÕńµ“£¬¾²ÖĆ”£Õńµ“Ē°ŗóµÄŹµŃéĻÖĻó_________(ĢīŃ”Ļī)”£
| A£®ÉĻ²ćŅŗĢå±ä±” | B£®ĻĀ²ćŅŗĢåŗģÉ«±äĒ³»ņ±äĪŖĪŽÉ« |
| C£®ÓŠĘųĢå²śÉś | D£®ÓŠ¹ūĻćĪ¶ |

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com