
��15�֣��ں� b molAlCl3����Һ�м��뺬 a mol NaOH����Һ��
��1������a��3b�������ι�����ʱ������Al��OH��3 ���������ʵ���Ϊ mol��
��2������a��4bʱ������Al��OH��3 ���������ʵ���Ϊ mol��
��3������3��a/b��4ʱ�����г������ɣ������в��ֳ����ܽ⣬��ʱAl��OH��3�����ʵ���Ϊ mol��
��4������д��AlCl3��NaOH��Һ��Ӧʱ�����йص����ӷ���ʽ��ѧ����ʽ��
�٣�
�ڣ�
�ۣ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13�֣�A��B��C��D��E��F���ֻ��������A��B��C��D��E���ɶ�����Ԫ����ɣ���ɫ��Ӧ��Ϊ��ɫ��B��C��E��������Ԫ����ɡ�B��C�����Ԫ����ͬ����C��Ħ��������B��80g/mol ���ش�
��1�����廯����AΪdz��ɫ��ĩ���û������к��еĻ�ѧ��Ϊ
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
���� �±�ΪB��Fʵ��IJ�������
| ���ں�B����Һ�м���ϡ |
| ��20mL��ˮ�еμ�F�ı�����Һ1~2mL����Һ��ʺ��ɫ |
| �۽�ʵ��ڵõ��ĺ��ɫҺ��������������գ����յõ�����ɫ���� |
д��B��ϡ![]() ��Ӧ�����ӷ���ʽ
��Ӧ�����ӷ���ʽ
д�����з�Ӧ�Ļ�ѧ����ʽ
��3������6������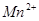 ��
�� ��
��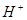 ��
�� ��
��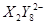 ��C�к��е������ӣ���
��C�к��е������ӣ���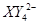 ���һ�����ӷ���ʽ����֪
���һ�����ӷ���ʽ����֪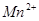 Ϊ��ԭ�����õ�1mol
Ϊ��ԭ�����õ�1mol![]() �������������ʵ���Ϊ mol
�������������ʵ���Ϊ mol
��4��������D��E�ת��D E������D��E ��
 H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ g��E ��
H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ g��E ��![]()
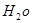 �Ļ�ѧʽΪ
�Ļ�ѧʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ӱ�ʡ��������ѧ�������ſ��Ի�ѧ�Ծ����������� ���ͣ������
��13�֣�A��B��C��D��E��F���ֻ��������A��B��C��D��E���ɶ�����Ԫ����ɣ���ɫ��Ӧ��Ϊ��ɫ��B��C��E��������Ԫ����ɡ�B��C�����Ԫ����ͬ����C��Ħ��������B��80g/mol ���ش�
��1�����廯����AΪdz��ɫ��ĩ���û������к��еĻ�ѧ��Ϊ
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
���� �±�ΪB��Fʵ��IJ�������
���ں�B����Һ�м���ϡ ������dz��ɫ���Ǻ�ʹ����ʯ��ˮ����ǵ���ɫ�д̼�����ζ������ ������dz��ɫ���Ǻ�ʹ����ʯ��ˮ����ǵ���ɫ�д̼�����ζ������ |
| ��20mL��ˮ�еμ�F�ı�����Һ1~2mL����Һ��ʺ��ɫ |
| �۽�ʵ��ڵõ��ĺ��ɫҺ��������������գ����յõ�����ɫ���� |
 ��Ӧ�����ӷ���ʽ
��Ӧ�����ӷ���ʽ 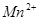 ��
��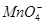 ��
�� ��
�� ��
��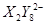 ��C�к��е������ӣ���
��C�к��е������ӣ��� ���һ�����ӷ���ʽ����֪
���һ�����ӷ���ʽ����֪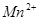 Ϊ��ԭ�����õ�1mol
Ϊ��ԭ�����õ�1mol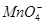 �������������ʵ���Ϊ mol
�������������ʵ���Ϊ mol E������D��E ��
E������D��E ��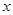 H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ g��E ��
H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ g��E ��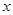
 �Ļ�ѧʽΪ
�Ļ�ѧʽΪ �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ�����и������ſ��Ի�ѧ�Ծ��������棩 ���ͣ������
��13�֣�A��B��C��D��E��F���ֻ��������A��B��C��D��E���ɶ�����Ԫ����ɣ���ɫ��Ӧ��Ϊ��ɫ��B��C��E��������Ԫ����ɡ�B��C�����Ԫ����ͬ����C��Ħ��������B��80g/mol ���ش�
��1�����廯����AΪdz��ɫ��ĩ���û������к��еĻ�ѧ��Ϊ
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
���� �±�ΪB��Fʵ��IJ�������
|
���ں�B����Һ�м���ϡ |
|
��20mL��ˮ�еμ�F�ı�����Һ1~2mL����Һ��ʺ��ɫ |
|
�۽�ʵ��ڵõ��ĺ��ɫҺ��������������գ����յõ�����ɫ���� |
д��B��ϡ ��Ӧ�����ӷ���ʽ
��Ӧ�����ӷ���ʽ
д�����з�Ӧ�Ļ�ѧ����ʽ
��3������6������ ��
�� ��
�� ��
��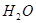 ��
�� ��C�к��е������ӣ���
��C�к��е������ӣ���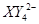 ���һ�����ӷ���ʽ����֪
���һ�����ӷ���ʽ����֪ Ϊ��ԭ�����õ�1mol
Ϊ��ԭ�����õ�1mol �������������ʵ���Ϊ mol
�������������ʵ���Ϊ mol
��4��������D��E�ת��D E������D��E ��
E������D��E �� H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ
g��E ��
H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ
g��E ��
 �Ļ�ѧʽΪ
�Ļ�ѧʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���������ʮ���и߶���ѧ�����п��Ի�ѧ���ģ��Ծ� ���ͣ������
��15�֣��ں� b mol AlCl3����Һ�м��뺬 a mol NaOH����Һ��
��1������a��3b�������ι�����ʱ������Al��OH��3 ���������ʵ���Ϊ mol��
��2������a��4bʱ������Al��OH��3 ���������ʵ���Ϊ mol��
��3������3��a/b��4ʱ�����г������ɣ������в��ֳ����ܽ⣬��ʱAl��OH��3�����ʵ���Ϊ mol��
��4������д��AlCl3��NaOH��Һ��Ӧʱ�����йص����ӷ���ʽ��ѧ����ʽ��
�٣�
�ڣ�
�ۣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com