
���� ��1���ȸ���ϡ��ǰ�����ʵ����ʵ������䣬�����Ũ��Һ�������ѡȡ���ʵ���Ͳ���ٸ�������һ�����ʵ���Ũ�ȵ���Һ��Ҫ��ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������
��2��������ȷʹ������ƿ�ķ��������жϣ�
��3������ƿ��©���������
��4������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1��������һ�����ʵ���Ũ�ȵ���Һ��Ҫ��ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������ȷ˳��Ϊ��EFGCHF��
�ʴ�Ϊ��EFGCHF��
��2��A������ƿ�Ǵ��л�����������ʹ��ǰҪ����Ƿ�©ˮ����A��ȷ��
B������ƿ������ˮϴ�����ܹ�ʹ�ô�����Һ��ϴ�����������Ƶ���ҺŨ��ƫ�ߣ���B����
C������ƿ�Ķ���������ֻ����������һ�����ʵ���Ũ�ȵ���Һ�����������ܽ⣬Ӧ�����ձ����ܽ⣬��C����
D���������Ƶ�������Һ�廹�ǹ��壬������������ƿ���ܽ⣬Ӧ�����ձ����ܽ��ϡ�ͣ���D����
E��������ɺ���Ҫҡ�����Ƶ���Һ������Ϊ���Ǻ�ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��ҡ����Σ���E��ȷ��
F����������ƿת��Һ��ʱ��Ҫ�ò���������������Һ����������ƿ���棬��F��ȷ��
��ѡBCD��
��3��������ƿ�м�ˮ�����߸������������ӣ�����ʳָ��ס���ӣ�����ָ�ⶥסƿ�ױ�Ե������2���ӣ��۲�ƿ����Χ�Ƿ���ˮ������ֱ����ת��ƿ��180�����ظ�һ�Σ���û��ˮ����˵��ƿ����©ˮ��
��4��A����Һ���������������ʣ�Ũ����ϡ�ͺ����ȵ�����Һ���¶ȸ������£��ܽ��ֱ��ת������ƿ�ᵼ��������Һ�����ƫС������������Һ��Ũ��ƫ�ߣ�
B��û�н�ϴ��Һת�Ƶ�����ƿ�У����ʵ����ʵ���ƫС������������Һ��Ũ��ƫ�ͣ�
C������ʱ���ӿ̶��ߣ����ƫС������������Һ��Ũ��ƫ�ߣ�
D������ʱ���ӿ̶��ߣ����ƫ����������Һ��Ũ��ƫ�ͣ�
E������Ͳ��ȡŨ���ᣬ����ʱ���ӿ̶��ߣ�����Ũ��������ʵ���ƫ��������Һ��Ũ��ƫ�ߣ�
��ѡ��ACE��
���� ������Ҫ����������һ�����ʵ���Ũ����Һ�IJ��������������������Ŀ�ѶȲ���ע�������ʵ���Ũ�Ⱥ������ٷ���֮��Ļ��㼰����ƿ��ʹ�÷�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
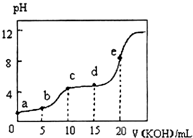 �����£���0.10mol•L-1KOH��Һ�ζ�10.00mL 0.10mol•L-1ij��Ԫ����H2R��Һ�����õζ�������ͼ��ʾ������������ȷ���ǣ�������
�����£���0.10mol•L-1KOH��Һ�ζ�10.00mL 0.10mol•L-1ij��Ԫ����H2R��Һ�����õζ�������ͼ��ʾ������������ȷ���ǣ�������| A�� | a����ʾ��Һ�У�$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$��1012 | |
| B�� | c����ʾ��Һ�У�c��K+����c��HR-����c��H2R����c��R2-�� | |
| C�� | e����ʾ��Һ�У�c��H+��=c��HR-��+2c��H2R��+c��OH-�� | |
| D�� | �������d��ʱc��HR-����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
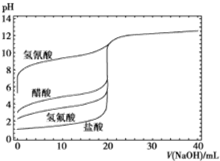 ��ͼ����0.1mol/LNaOH��Һ�ֱ�ζ�20mLŨ�Ⱦ�Ϊ0.1mol/L�IJ�ͬһԪ��ĵζ����ߣ�����˵��������ǣ�������
��ͼ����0.1mol/LNaOH��Һ�ֱ�ζ�20mLŨ�Ⱦ�Ϊ0.1mol/L�IJ�ͬһԪ��ĵζ����ߣ�����˵��������ǣ�������| A�� | ���ԣ�HF��CH3COOH��HCN | |
| B�� | ��NaOH��Һ�ζ�����ʱ��Ӧ�÷�̪��ָʾ������ʹ�ü��� | |
| C�� | ������10mLNaOH��Һʱ��c��CN-����c��CH3COO-�� | |
| D�� | ��NaOH��Һ�ĵ��룬CH3COOH��Һ��ˮ�ĵ���̶��ȱ����С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{1}{n}$ mol-1 | B�� | 9n mol-1 | C�� | 2nmol-1 | D�� | 18nmol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Al3+�Ľṹʾ��ͼ�� | B�� | �Ȼ��Ƶĵ���ʽ��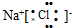 | ||
| C�� | �����ӵĵ���ʽ��Al3+ | D�� | ��ԭ�ӵĽṹʾ��ͼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ�У�K+��MnO4+��SO42-Cl- | |
| B�� | ��ʹ�����Ժ�ɫ����Һ��Fe2+��NO3-��Na+��SO42- | |
| C�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=l0-12��ˮ��Һ�У�NH4+��Al3+��NO3-��Cl- | |
| D�� | ��������Һ�У�Cu2+��Al3+��SO42-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
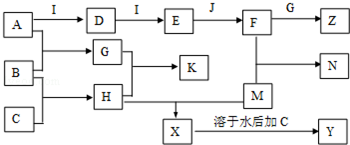
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
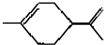 �������й������Ʋⲻ��ȷ���ǣ�������
�������й������Ʋⲻ��ȷ���ǣ�������| A�� | ��ʹ���Ը��������Һ��ɫ | |
| B�� | ������Ϊ��̬��������ˮ | |
| C�� | ����ʽΪC10H18 | |
| D�� | ����������CCl4��Һ��Ӧ�����Ϊ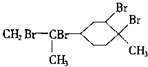 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com