
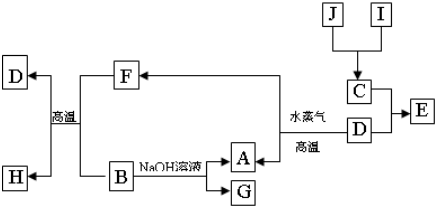
A��B��C��D�����ֳ����ĵ��ʣ�A��BΪ������C��D�����������壬��DΪ����ɫ���塣�ס��ҡ���Ϊ�����Ļ�������Ǻ�ɫ�Ҿ��д��Ե����ʡ�����֮���ת����ϵ����ͼ��ʾ��

��ش��������⣺
��1��B���Ӧ�Ļ�ѧ����ʽ�� ��
��2�������£���A��B�ĵ��ʷ���Ũ�����Ũ�����У��Ƿ��ܽ⣿ ����ǡ�����
��3����������ˮ�����Һ��������������ӵķ�����
��
��4��д��A��ˮ������Ӧ����C�ͼĻ�ѧ����ʽ ��
��5����A��B���ֽ�����һ������������ɻ���
��ȡһ�������ĸû��������м���������NaOH��Һ���������������ڱ�״����Ϊn L��B��NaOH��Һ��Ӧ�����ӷ���ʽ�� ���������B�����ʵ���Ϊ mol���ú���ĸ�ķ���ʽ��ʾ����
����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����Ϊm L���÷�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ mol���������A������Ϊ g���ú���ĸ�ķ���ʽ��ʾ����
��������õ���Һ�м������������������Һ����ֽ��裬�������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȣ���ԭ�������������������Ϊ ��
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
 Al��OH��3+OH-
Al��OH��3+OH- Al��OH��3+OH-
Al��OH��3+OH-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ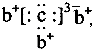 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������| A��ԭ�Ӱ뾶��a��c��d��b | B����ۺ����������c��d��a | C��ԭ��������a��d��b��c | D�����ʵ�������a��b��d��c |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com