
 ijͬѧ������100mL 0.10mol/L CuSO4��Һ�����²���1��5�������ƹ��̼�ʾ��ͼ��
ijͬѧ������100mL 0.10mol/L CuSO4��Һ�����²���1��5�������ƹ��̼�ʾ��ͼ��| n |
| V |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������е���AgNO3��Һ�������е���Ԫ�أ�Br-+Ag+�TAgBr�� | ||||
| B���ô����ȥˮ����CaCO3+2H+�TCa2++H2O+CO2�� | ||||
C��������������Լ��ķ�Ӧ��CH2OH��CHOH��4CHO+2Cu��OH��2+OH-
| ||||
D����������Һ��ͨ������CO2��CO2+H2O+ �� �� +HCO3- +HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ͬ����Ԫ����X�Ľ�������ǿ |
| B��ͬ����Ԫ����Y����ۺ������������ǿ |
| C��ԭ�Ӱ뾶X��Y�����Ӱ뾶X+��Z2- |
| D��ͬ��Ԫ����Z���⻯���ȶ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��7 | B��8 | C��9 | D��10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� I2��g��+H2��g��?2HI��g�� |
B��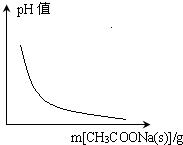 CH3COOH?H++CH3COO-��������Һ����仯�� |
C��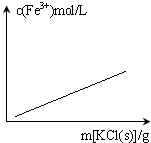 FeCl3+3KSCN?Fe��SCN��3+3KCl������Һ������仯�� |
D�� CH3OCH3��g��+3H2O��g��?6H2��g��+2CO��g��-Q����ѹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��7.1g����������������������Һ��Ӧת�Ƶĵ�����Ϊ0.2��6.02��1023 |
| B��V L a mol?L-1���Ȼ�����Һ�У���Fe3+����ĿΪ6.02��1023����Cl-����Ŀ����3��6.02��1023 |
| C����ҵ�õ�ⷨ���д�ͭ����ʱ��ÿת��1mol���ӣ��������ܽ��Cu����Ϊ0.5��6.02��1023 |
| D����״���£�22.4LNO��11.2L O2��Ϻ�����ķ�������Ϊ1.0��6.02��1023 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com